Journal of
eISSN: 2373-4396


Research Article Volume 15 Issue 5
1Department of Cardiology, Hospital São Lucas, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Brazil
2Department of Cardiology, Instituto Dante Pazzanese de Cardiologia, Brazil
Correspondence: Andrés Di Leoni Ferrari, Cardiac Stimulation Unit, Cardiology Department, Hospital São Lucas, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Av. Ipiranga 6690, Brazil, 90610-000, Tel 55-51-33205023
Received: October 30, 2022 | Published: December 27, 2022
Citation: Ferrari ADL, Bartholomay E, Velho FM, et al. Atrioventricular dyssynchrony in patients with permanent pacemaker due to sinus node dysfunction and first-degree atrioventricular block: does the long PR syndrome exist? J Cardiol Curr Res. 2022;15(5):124-131. DOI: 10.15406/jccr.2022.15.00567
Background: First-degree atrioventricular block (AVB) might not be benign. Markedly long PR intervals may cause cardiac dyssynchrony, with many consequences. Restoring optimal AV synchrony represents a reasonable option for hemodynamic and clinical improvement.
Objectives: To compare 2 cardiac pacing strategies for bradycardia associated with first- degree AVB: (1) long PR interval (PRi)–narrow intrinsic QRS, avoiding ventricular pacing but potentially causing AV dyssynchrony (AVD); vs (2) optimized AV interval (oAVi)– wide paced QRS, potentially inducing ventricular dyssynchrony.
Methodology: Prospective cohort study with patients with permanent DDD pacemakers due to sinus disease associated with first-degree AVB (binodal disease). We analyzed diastolic filling time (DFT), defining 2 groups: patients with AV synchrony (AVS) and AVD. Clinical and echocardiographic follow-up was performed for a year.
Results: We studied 43 patients (mean age 71 years; 51.2% female). Longer PRis were associated with worse baseline ventricular systolic function. The AVD group (24/43) showed longer PRi (mean=283.5ms; p≤0.001) and reduced ventricular DFT (p=0.032). First-degree AVB with PRi>263ms (relative risk [RR]=1.84; p=0.024; specificity=78.9%; 95% confidence interval [CI] 0.43–0.79) and DFT<40% of the cardiac cycle duration (RR=0.99; p<0.001) were independent predictors of AVD. When PRi>300ms, dyssynchrony was not correctable by AVi optimization. The AVS group (controls, n=19; mean PRi=252.4ms), despite maintaining synchrony, had worsened mitral regurgitation (p=0.008) at follow-up.
Conclusions: First-degree AVB comprehends significantly different patients: those with AVD and AVS, determined by DFT and PRi length. In those with AVD, we hypothesized the existence of the “long PR syndrome”, defined from a PRi>263ms associated with overt DFT impairment.
Keywords: artificial cardiac pacemaker, diastolic disfunction, first-degree atrioventricular block, AV conduction, long PR interval
SND, sinus node dysfunction ; AVB, atrioventricular block; PRi, PR interval; ACP, artificial cardiac pacing; RV, right ventricle; AVi, atrioventricular interval; AF, atrial fibrillation; HF, heart failure; ECG, electrocardiogram; DFT, diastolic filling time
Binodal disease is characterized by an association of sinus node dysfunction (SND) and atrioventricular block (AVB).1 When SND is treated with the implantation of a permanent dual-chamber pacemaker (PPM) and AVB manifests as first-degree AVB (PR interval [PRi] >200ms), a question arises: which atrioventricular interval (AVi) should be programmed so that AV dyssynchrony is corrected, the right ventricle (RV) is paced, and pacing-induced cardiomyopathy is avoided?
Conventional artificial cardiac pacing (ACP) is characterized by the apical implantation of a ventricular lead, imposing an anti-physiological electrical pattern, similarly to what happens during left bundle branch block (LBBB). RV apical pacing produces inter and intraventricular dyssynchrony, which is associated with worsening systolic function, atrial fibrillation (AF), and heart failure (HF).2 PPM devices hence have algorithms and settings that aim to avoid RV pacing, prioritizing ventricular depolarization through the intrinsic conduction system and narrow QRS; paradoxically, artificially long PRi are accepted, which are probably also anti-physiological.1–4
Cardiac dyssynchrony is a difference in the timing of electrical and mechanical activation of the heart, which negatively affects cardiac efficiency.1 In this context, loss of AV synchrony (AVS) is suspected when an excessively prolonged PRi is observed at the surface electrocardiogram (ECG) (first-degree AVB), associated with symptoms due to the uncoupling of physiological coordination between atrial contraction and ventricular filling and systole. Mechanically, there is an early and incomplete closure of the mitral valve, frequently associated with the development of mitral regurgitation (MR).
Fundamentally, this AV decoupling due to the long PRi shortens diastolic filling time (DFT), with a negative effect on preload, affecting systolic function, and triggering or worsening presystolic MR.5
Population
Patients were included with an indication for dual-chamber PPM implantation due to irreversible symptomatic bradycardia (heart rate [HR] <60bpm), characterizing SND, and who under DDDR programming with an RV pacing minimization algorithm demonstrated PRi >200ms (first-degree AVB), suggesting binodal impairment. We excluded patients with reduced left ventricular ejection fraction (LVEF) (<50%), chronic or persistent AF in the previous year, patients with second- or third-degree AVB, prosthetic valves, poor acoustic window at transthoracic echocardiography (preventing measurements), or a QRS interval ≥130ms (in DI, DII, and V1 leads), whether it be intrinsic or after surgical positioning of the RV lead (paced QRS). We also excluded patients who maintained paced QRS (absence of intrinsic rhythm) after adjusting for maximal AVi, or with a life expectancy of less than 1 year. The protocol was approved by the Hospital’s Ethics Committee (11/05664) and in all cases written informed consent was provided.
Study protocol
All patients received Accent® devices (Saint Jude Medical, Sylmar, CA, USA), with the RV lead positioned on the upper region of the interventricular septum (para-hisian pacing) and confirmed through radiography in anteroposterior and left oblique views, and with the atrial lead on the right atrial appendage. The PPM was adjusted according to individualized indications. For echocardiographic assessment of AVD, during the study protocol atrial pacing was set at 10 bpm above the native sinus rate. This way we observed, with an intrinsic conduction setting (long PRi–narrow QRS), the pA–sV sequence (paced atrium–sensed ventricle), whereas when using DDD pacing to determine optimal AVi (optimized AVi–paced QRS), we observed the pA–pV sequence (paced atrium–paced ventricle).
We used the following definitions
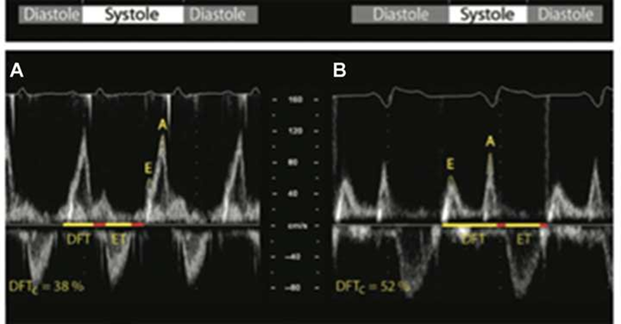
Figure 2 Optimal AV interval (oAVi) is defined when allowing the completion of the atrial contribution to diastolic filling (diastolic filling time [DFT], which should last for at least 40% of the cardiac cycle). The oAVi results in a more favourable preload before ventricular contraction, with minimal mitral regurgitation.
Left: AV dyssynchrony (A), shown by fused and almost superposed E and A waves at Doppler transmitral flow, determining their sum to result in suboptimal DFT (38%), not allowing the conclusion of the atrial contribution to ventricular.
Right: oAVi (B), the sum of E and A wave durations spans 52% of the cardiac cycle, resulting in favourable preload before ventricular contraction [1]. DFTc, DFT corrected for RR interval; ET, ventricular ejection time; E and A, waves at Doppler transmitral flow.
According to our protocol, patients with AVD were divided into subgroups: those who could not restore synchrony (uncorrected AVD) and those who became synchronic after AVi intervention (optimization) (corrected AVD). Uncorrectable AVD patients (UAVD) were those who despite optimization attempts, had the AVi shortened to 120ms, and we could not verify any of the prespecified conditions for AVS. In these cases, follow- up was performed with the AVi settings that resulted in the best VTI. The remaining patients were considered to have correctable AVD (CAVD).
Follow-up
The AVS group maintained a long PRi–baseline intrinsic QRS upon a positive AVi hysteresis algorithm (Ventricular Intrinsic Preference VIP® Saint Jude Medical). The AVD group received an optimized AVi setting, where the best hemodynamic performance was obtained as a function of AVS restoration (oAVi–paced QRS). In all cases, after 3, 6 and 12months (as per the predetermined 1-year follow-up), patients were reassessed with transthoracic echocardiography through cardiac chamber diameters and LVEF measurements. Six months after inclusion, all patients with AVD returned to baseline PRi (long PRi–intrinsic QRS crossover) under the VIP® algorithm (Figure 3). The PPM event monitor (Holter) was used to assess the incidence of arrhythmias.
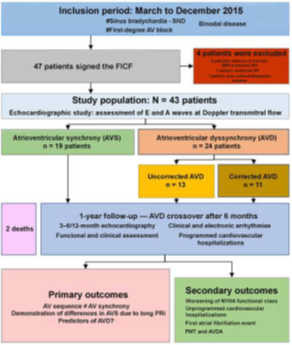
Figure 3 Study protocol. Recruitment, inclusion, follow-up, and outcomes.
SND, sinus node disease; AV, atrioventricular; FICF, free and informed consent form; NYHA, New York Heart Association; PMT, pacemaker-mediated tachycardia; AVDA, AVD-induced arrhythmia.
Statistical analysis
Quantitative data were presented as mean ± standard deviation, and categorical data as absolute and relative frequencies. Shapiro-Wilk test was used for assessing data normality. Pearson’s chi-squared or Fisher’s exact tests were applied for comparing categorical variables between groups. Student’s t-test for independent samples was used for comparing continuous variables with symmetrical distribution between groups. The generalized estimating equation (GEE) model was chosen for comparing parameters over time, and the Bonferroni test was applied for identifying differences between groups. The association between PRi increases and LVEF reduction was established through Pearson’s correlation analysis. Poisson regression analyses were used for determining predictors of AVD. The criterion for including variables in the multivariate model was based on the literature and biological plausibility. For determining the best cut-off point for diagnosing AV dyssynchrony using PRi, we used the receiver operating characteristic (ROC) curve and prioritized specificity results. The analysis of follow-up free of AF events was performed using the Kaplan-Meier method and results were compared through the log-rank test. A p value < 0.05 was considered an indicator of statistical significance. We used SPSS software v.17.0 for our analyses (SPSS Inc., Chicago, IL, USA).
Main characteristics of the studied population are presented on Table 1. The different phases of our study are presented on Figure 3. We analyzed 19 patients in the AVS group and 24 patients in the AVD group. The mean age of our patients was 71.5 years with a slight female predominance (51.2%), which was higher in the AVS group (p=0.08). A PRi value of 262.5ms (263ms) had the best discriminatory ability to diagnose AVD in the studied population (specificity: 78.9%, sensitivity: 58.3%; area under the ROC curve: 0.61; 95% confidence interval [CI]: 0.43–0.79).
Variables |
Total sample N = 43 |
AV synchrony (SAV) n = 19 |
AV dyssynchrony (DAV) n = 24 |
p-value |
Age (years) |
71.5±12.2 |
73.2±13.4 |
70.3±11.2 |
0.44 |
Sex Female |
22 (51.2%) |
13 (68.4%) |
9 (37.5%) |
0.088 |
Pre-existing heart disease |
0.26 |
|||
Ischemic |
10 (23.3%) |
6 (31.6%) |
4 (16.7%) |
|
Other |
2 (4.7%) |
0 (0.0%) |
2 (8.3%) |
|
No heart disease |
31 (72.1%) |
13 (68.4%) |
18 (75.0%) |
|
FC I |
35 (23.3%) |
17 (89.5%) |
18 (75.0%) |
0.27 |
FC II |
8 (18.6%) |
2(10.5%) |
6 (25.0%) |
|
Medication β-blocker |
13 (30.2%) |
5 (26.3%) |
8 (33.3%) |
|
Antiarrhythmic |
4 (9.3%) |
1 (5.3%) |
3 (12.5%) |
0.62 |
Others (diuretics, ASA, ARBs, thiazides) |
21 (48.8%) |
10 (52.6%) |
11 (45.8%) |
0.89 |
Table 1 Demographic and clinical characteristics of patients in the study
Age is presented as mean±standard deviation. Other variables are presented as absolute numbers (n) and corresponding percentages.
AV, atrioventricular; SAV, synchronic AV; DAV, dyssynchronic AV; FC, functional class; NYHA, New York Heart Association; ASA, acetylsalicylic acid; ARBs, angiotensin receptor blockers
The AVD group showed, as particularly relevant findings due to their clinical impact, prolonged PRi (mean duration 283.5ms ± 61.2ms; p=0.032) associated with a reduction in DFT (16% shorter; p<0.001) and a significant decrease in LVEF as the PRi increased (Figure 4). The UAVD subgroup constituted the majority (13/24) of AVD cases and showed the longest PRi and significantly (p=0.001) higher electromechanical impairment due to reduced DFT.
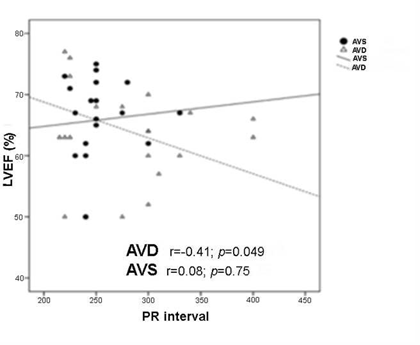
Figure 4 Association between PRi increase and left ventricle ejection fraction (LVEF) reduction. A significant worsening of systolic function is demonstrated in the AVD group with longer PRis. AVD, AV dyssynchrony; AVS, AV synchrony.
Regarding β-blockers, despite their influence on AV conduction and PRi prolongation, a translation into outcomes was not proven in this study. No significant difference was observed between groups regarding the incidence of the other predefined outcomes, but among arrhythmic consequences of AVD, we verified an expressive incidence of AF during follow-up: two-thirds of the patients in our population presented at least one documented episode, suggesting an association between long PRi and AF occurrence. Moreover, we noticed a 30-day difference (median in the AVS group = 49 days, vs 79 days for AVD; p=0.174) between groups until the first event.
Electronic arrhythmias had an important participation (Table 2): both pacemaker- mediated tachycardia (PMT) and an even more prevalent form, named AVD-induced arrhythmia (AVDA). With statistical and clinical importance and specifically comparing those who developed AVDA against those who did not present it, regardless of the group (AVS or AVD), patients with this peculiar electronic arrhythmia presented more sustained AF episodes (p=0.039 vs non-AVDA).
Variables |
Total sample N = 43 |
AV synchrony (SAV) n = 19 |
AV dyssynchrony (DAV) n = 24 |
p-value |
PMT |
16 (37.2%) |
9 (47.4%) |
7 (29.2%) |
0.36a |
AVDA |
8 (18.6%) |
4 (21.1%) |
4 (16.7%) |
1.00a |
Atrial fibrillation |
29 (67.4%) |
15 (78.9%) |
14 (58.3%) |
0.27a |
Pacemaker syndrome symptoms |
5 (11.6%) |
3 (15.8%) |
2 (8.3%) |
0.64a |
Hospitalizations |
12 (27.9%) |
5 (26.3%) |
7 (29.9%) |
1.00a |
Table 2 Outcomes in the studied sample, per group
Values are presented as absolute numbers (n) and corresponding percentages. a: Fisher’s exact test.
AVS, AV synchrony; AVD, AV dyssynchrony; AV, atrioventricular; PMT, pacemaker- mediated tachycardia; AVDA, AVD-induced arrhythmia.
For better understanding differences in the context of AV dyssynchrony, the PRi was stratified into 3 levels: extreme increase if the PRi exceeded 300ms, moderate increase if it was between 262.5ms and 300ms, and mild if it reached 200ms to 262.4ms. By this analysis, the AVS group showed PRis that predominantly fell into the mild increase range; moderate increases included mostly patients with CAVD and, finally, the extreme increase range included mostly patients with UAVD. In the UAVD subgroup, 7 patients (16.3% of the sample) had more severe diastolic dysfunction and, in comparison with the others, were more symptomatic (as expected) (p=0.049).
Importantly, patients in the AVD group tended to improve LVEF under optimized AVi, despite paced QRS. Even more remarkable was the new observed negative impact on LVEF when returning to baseline PRi during crossover (Figure 5). In the AVS group (long PRi–intrinsic QRS), our results suggest worsening of mitral valve function, manifested as MR. At baseline, MR was present in 63.2% of the patients, while at the end of follow-up (1 year), MR was detected in 84.6% of them.

Figure 5 LVEF progression during follow-up after AVD correction. Increasing trend under the influence of oAVi and wide QRS in the AVD group. AVD, AV, dyssynchrony; AVS, AV synchrony.
Of paramount importance to this study, PRi over 263ms (RR 1.84; p=0.024) and a reduction in DFT (<40% of the cardiac cycle) determined by the sum of E and A waves at Doppler transmitral flow (RR 0.99; p<0.001) were predictors associated with AV dyssynchrony (Table 3). Similarly, we verified that, for every adjusted 1ms in DFT (AV optimization), there was a 1% reduction in the probability of AV dyssynchrony. Tangentially, our findings suggest that men would have a higher tendency to lose AVS when under long PRi (RR 1.63; 95% CI 0.95–2.81; p=0.079).
Variables |
RR (95% CI) |
p-value |
AV conduction disorder PRi >263ms |
1.84 (1.09–3.12) |
0.024 |
Diastolic dysfunction DFT <40% of the cardiac cycle |
0.99 (0.98–0.99) |
< 0.001 |
Table 3 Independent predictors of AV dyssynchrony through long PRi
Sum of E and A wave durations at Doppler transmittal flow.
This study demonstrates that AV “sequence” (mainly a series of electrical events) is different from AV “synchrony” (electromechanical phenomena) in the first-degree AVB scenario and sets the stage for debating the existence of a “long PR syndrome.”
It is established that minimizing AV pacing by keeping long PRi pursuing a narrow QRS is efficient and can preserve ventricular mechanical synchrony while preventing pacing-induced cardiomyopathy.9–11 Our main finding was the diagnosis of individuals with binodal disease who did not benefit from this strategy due to the resulting AV uncoupling and dyssynchrony. In these specific patients, when the PRi exceeded 263ms, we observed an 84% increase in the risk of AVD. Therefore, it is possible to answer our proposed question about whether a long PRi or a paced QRS should be preferred: there is a subpopulation in which maintaining the “AV sequence” under prolonged PRi does not mean AVS and does not always translate into mechanical, hemodynamic, or functional cardiac benefits.
It is thus proven that, in patients with AVD, there was a higher incidence of significantly longer PRi, and that patients with both long PRi and more severe AVD showed significantly reduced baseline systolic function. From these data, we infer that as the PRi increases further away from values considered normal, the efficiency of the systolic cardiac pump significantly decreases (Figure 4) probably due to electromechanical AV dysfunction. Still in the AVD group, during follow-up with optimized AVi, we observed a trend towards LVEF protection against MR and a delayed first AF event, which is attributable to the hemodynamic recovery promoted by the restoration of AVS despite the action of the paced QRS.
The positive impact of AVS restoration by an optimal AV interval achieved with dual-chamber pacing was corroborated when, after returning to baseline PRi at the 6-month crossover, systolic function was again decreased, which was attributable to AVD resurgence.
Although historically considered benign, many recent publications associate first- degree AVB with worse prognosis, both in the general population and individuals with cardiovascular disease.12–16 A meta-analysis with 328932 individuals confirmed that an increase in PRi duration is an independent risk factor for AF.17 The Framingham heart study showed that patients with first-degree AVB had higher all-cause mortality, twice the risk of developing AF, and three times the risk of requiring PPM implantation when compared to the general population.13 Every 20ms increase in PRi was associated with an adjusted risk ratio of 1.11 for AF, 1.22 for requiring PPM implantation, and 1.08 for all- cause mortality.18
Briefly, one can say that cardiac pacing solves the electrical and hemodynamic cardiac issue, but it does so at the expense of potential mechanical consequences to the heart that translate to the clinic (AVD, HF, AF, and others).1,19–21 Current literature recommends, for patients with SND who have preserved 1:1 AV conduction, the association of strategies for minimizing artificial ventricular pacing.22–26 However, Healey et al.17 after analyzing more than 7000 patients included in randomized studies, showed that atrial-based ACP (prioritizing narrow QRS) constitutes a favorable environment for the occurrence of arrythmias, especially AF, when compared to DDDR, as also proven in our study. Therefore, despite the enthusiastic promotion of the benefits of different methods for reducing RV pacing as a whole, one can verify that, when tested in specific clinical settings such as this study, these mechanisms provide results that tend to be at least arguable.9 This raises questions on which is the best programming and operation setting for patients with PPM due to binodal disease.1
Our results suggest that a review of current guidelines is needed27,28 considering the indication of ACP for patients with SND and first-degree AVB (binodal disease profile). In the past, the focus was only on hemodynamic aspects, but now it should be considered that cardiac derangement due to prolonged PRi has a diversity of heterogeneous contributions, many of them treatable elements of the dyssynchrony process. The restoration of an optimal AV synchrony, achieved with near physiological DDD pacing, may represent a reasonable therapeutic option. Although there is low evidence to support that ACP improves survival in patients with isolated first-degree AVB, it was proven in this study that extreme PRi (as demonstrated, the effects start at 263ms) may trigger hemodynamic effects that are capable of causing AV dysfunction, AF, and MR. An early atrial systole would induce diastolic reversal of mitral flow (diastolic MR) and the development of a ventriculoatrial pressure gradient, resulting in early diastolic closure of the mitral valve. This “atrium-induced” closure may not be complete, and the mitral valve may reopen if atrial contraction is not followed by an adequately timed ventricular systole. This would explain many of the symptoms and clinical and functional manifestations associated with AVD.8 This is why some authors suggest certain clinical and functional improvements in patients with PRi > 300ms, when subjected to DDD pacing under AVi optimization.12,29,30
In light of our results, the arbitrary 300ms limit established in the literature12,27,28 as a cut-off point for consequences of long PRi and indication for PPM implantation may constitute a pragmatic simplification of a much more complex electromechanical pathophysiology. With relevant specificity (78.9%), we demonstrated that a PRi >263ms signaled the beginning of a risk of diastolic dysfunction and its consequences. Starting from this threshold, we verified the appearance of different degrees of electromechanical AV derangement. However, according to our findings, if the PRi exceeds 300ms, some patients’ AVD is so severe that it would not be correctable by the echocardiographic optimization of AVi (UAVD subgroup).
In association with an AVD diagnosis, we found a significant risk of clinical arrhythmias, predominantly AF, but also of other pacemaker-mediated arrhythmias. In patients with binodal disease, atrial pacing may induce an abnormal increase in AV conduction time through long PRi, establishing an appropriate environment for the occurrence of “electronic” reentry-mediated arrhythmias.9 PMT is the most commonly known, but not the only one of this kind. We observed another peculiar electronic arrhythmia, named AVDA.1,12,31
In this context, more apparent than the physiological role of maintaining HR (Cardiac Rate) by the PPM are the alterations due to the electronic dysfunction due to the long PRi, capable of triggering ectopic activation, which depending on the moment and circumstances may induce arrhythmias. PPMs have timing software that limits operating intervals, regulating the stimulation function while processing signals detected in the myocardium (sensing function). A common strategy for approaching PMT is to prolong the post-ventricular atrial refractory period (PVARP), and the main difference between AVDA and PMT is that, in the presence of both a long PRi and extended PVARP, in case of high HRs the next P wave will be “pushed” into the refractory period or will be retained within the previous cycle of the atrial channel (post-ventricular atrial blanking). The P wave occurring in these intervals will not be processed and will be considered electronically inexistent by the PPM. If the P wave of the next beat comes too early, in addition to an electromechanical impairment of AVS, an atrial pace may reach the atrial tissue during repolarization and increases the risk of AF arrhythmias, as corroborated in this study.31
The observed difference between median values (49 vs 79 days) until the first AF event demonstrates the importance of a theoretical protective mechanism by correcting dyssynchrony in the AVD group (AV optimization) and reinforces the hypothesis of the participation of mechanical alterations in the genesis of arrhythmic events.1 It is also pertinent to differentiate this particular kind of AV dysfunction from the electronic events of pacemaker syndrome. Due to the long PRi, the closer the atrial systole is to the previous ventricular systole (P-T fusion, Figure 6), the same clinical consequences of retrograde ventriculoatrial conduction would be produced, as well as manifestations that are similar to those of the classical pacemaker syndrome.32,33 In this case, however, AVD is purely systolic because the atrium and ventricle contract simultaneously, commonly associated with the VVI mode in the presence of sinus rhythm.33 Conversely, our findings justify AVD as systo-diastolic. Long PRis produce hemodynamic alterations due to electromechanical AV decoupling, where left and right ventricular filling is compromised because the atria contract before the AV valves open, which initiates ventricular diastole.1
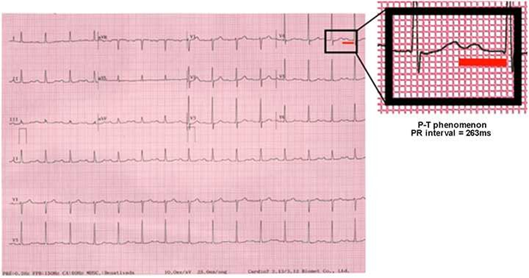
Figure 6 A significant risk of AVD is verified when the 263ms threshold is exceeded and the “P over T” phenomenon is observed; the occurrence of long PR syndrome may be suggested..
From our research, we highlight the importance of considering the equilibrium between AVD due to long PRi (a result of atrial pacing for chronotropic support, or jointly to the action of algorithms for reducing RV pacing) and intra/interventricular dyssynchrony due to paced QRS. Restoring optimal AV synchrony with dual-chamber physiological pacing may represent a reasonable therapeutic option leading to hemodynamic and clinical improvement. However, in all cases, the recommendation for optimizing AVi should be confronted with the potential risk of pacing-induced cardiomyopathy.1 Hypothetically, the potential prejudice caused by ACP could, in this protocol, have been dampened by the fact that artificial ventricular activation was obtained from endocardial areas of the RV that are closer to the native conduction system. In this study, the ventricular lead was positioned on the uppermost portion of the interventricular septum and probably allowed a fast, nonselective capture of the physiological system (para-hisian); profiting from the complex natural electrical distribution network, it provided closer to intrinsic ventricular pacing (QRS duration <130ms).34 The favorable progression of LVEF verified in the AVD group demonstrates the plausibility, in well-selected individuals, of correcting AVD without the negative impact of paced QRS when optimizing AVi from this pacing site. This would be conditioned to ventricular lead positioning in a region where artificial activation determines a QRS that, although different from native, comes closer to natural cardiac activation.1,34–37
The premise for using the intrinsic conduction system (or getting as close to it as possible) opens new possibilities for future studies, especially considering the revision of guidelines for indicating PPM implantation for first-degree AVB and for programming devices in binodal disease. In this sense, new horizons are in constant expansion, since with this strategy (physiological pacing) it would probably be possible to maintain or restore AVS under paced QRS and without the fear of potential injury inflicted by the negative impact of traditional RV pacing.1,2,34,35
With favorable evidence regarding the results of near physiological pacing strategies34,35,37–39 (which were not the aim of the study), we reinforce the notion that patients subjected to AV optimization with this methodology have less RV remodeling and structural cardiac damage and superior LVEF preservation when compared with those under conventional (apical) pacing.34–39 No matter the hypothesis, a favorable perspective is presented for avoiding a situation where a choice would be made (Figure 1) between one dyssynchrony or the other (AV due to long PRi or inter/intraventricular due to paced QRS).
A complementary explanation to the absence of a potential negative repercussion associated with RV pacing in our sample lies on the substrate. Since these are patients with no structural cardiac alterations and normal LVEF, they would initially be more affected by AVD due to long PRi than due to paced QRS. Appropriate electromechanical timing derived from AVi optimization would offset, for a certain period (not determined in this study, but certainly longer than 1 year), potential negative consequences of ventricular pacing. This observation is in line with the results of an analysis of the MOde Selection Trial (MOST),40 where after a mean follow-up of 33.1months it was demonstrated that only 10% of patients with preserved LVEF and no structural heart disease developed pacing-induced HF.
Finally, after a thorough analysis of our results and grouping the characteristics of patients with AVD, mainly determined by the PRi <263ms and compromised diastolic filling (DFT <40% of the cardiac cycle), the existence of a “long PR syndrome” is put into perspective. It would consider a heterogeneous population that stands out by its clinical and electromechanical AV derangement, with repercussions to ventricular function determined by a permissive maintenance of anti-physiological long PRi (> 263ms).1,2
Hemodynamically, it would group together LVEF findings that worsen as PRi increases and are corrected by optimizing AVi. From an electrocardiographic point of view, it associates a “P over T” phenomenon and long PRi with higher incidence of arrhythmias (both electronic and AF), and structurally, it exposes MR worsening over time. Diastolic MR may significantly contribute, in the wider context of long PR syndrome, to unfavorable hemodynamic circumstances in patients with first-degree AVB (Figure 7).
The paper has the expected limitations for a study with a small, very selected population (binodal disease, preserved LVEF, almost normal paced QRS, devices from the same manufacturer, etc) which was not randomized and was analyzed during a short follow-up period. Although uniform criteria contribute to reducing measurement bias, the lack of a core laboratory for analyzing echocardiographic images constitutes a limitation and could influence some of the affirmations extracted from our data. At the same time, and emphasizing the complexity of the involved aspects, it is unknown if long native PRi or paced oAVi change with variations in positioning, throughout the day, or if the AVD condition could be modulated by other clinical situations such as increased HR during physical activity, which was not included in the objectives of this study Finally, it is timely to state that the theory of long PR syndrome, under the hypothesis of AVD and without sounding pretentious, aims to didactically organize new observations for clinical practice, facilitating the comprehension of an important issue.
We demonstrated significantly distinct characteristics in patients with first-degree AVB associated with SND (binodal disease): AVD and AVS. The differences are determined by PRi duration when it exceeds 263ms (Figure 6) and by compromised DFT (<40% of the cardiac cycle), denoting diastolic dysfunction and dyssynchrony (Figure 2). In this specific population, a benefit of optimizing AVi associated with positive effects of DDD pacing from alternative, more physiological sites (para-hisian or selective conduction system pacing) could still exist. Nevertheless, AVD in binodal disease as the manifestation of a possible long PR syndrome should still be better studied and its existence should be confirmed by further clinical studies.
None.
None declared by the authors relevant to this article.
None declared by the authors relevant to this article.

©2022 Ferrari, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.