Journal of
eISSN: 2373-4396


Research Article Volume 14 Issue 6
Cardiology Department, Zagazig University Hospital, Egypt
Correspondence: Ragab A Mahfouz, Professor of Cardiology, Zagazig University Hospital, Egypt, Tel 00201006427671
Received: October 05, 2021 | Published: November 23, 2021
Citation: Mahfouz RA, Gad MM, Arab M, et al. Aortic root systolic excursion and its association with exercise tolerance in patients with heart failure with preserved ejection fraction. J Cardiol Curr Res. 2021;14(6):155-161. DOI: 15406/jccr.2021.14.00533
Objective: We aimed to investigate the association between aortic root systolic excursion (ARSE) as a simple echocardiographic parameter and exercise tolerance in patients with heart failure with preserved ejection fraction (HFpEF).
Methods: Eighty patients (mean age 57.9±10.5years) with HFpEF were compared with 80 with age and sex matched healthy subjects. Transthorathic echocardiography was performed with specific assessment of aortic root systolic excursion. Left ventricular longitudinal (LVGLS) and circumferential strain (LVGCS) were evaluated with speckle tracking imaging. In addition all participates underwent 6minute walking test (6MWT).
Results: ARSE was reduced in subject with HFpEF compared with controls (p<0.05). Furthermore, HFpEF patients with 6MWTD<300m had pronounced decrease in ARSE compared with those with 6MWTD≥300m and control subjects (p<0.001). 6MWTD was correlated with ARSE (p<0.001), LVGLS (p<0.001) and LVGCS (p<0.01). ARSE had significant correlation with LVGLS (p<0.001) and LVGCS (p<0.003). Moreover, ARSE correlated negatively with LAVI (r =-0.438, p<0.001), E/e' ratio (r =- 0.349, p<0.01). After multivariate analysis ARSE remained a strong independent predictor of exercise tolerance in patients with HFpEF (p<0.001). ROC analysis revealed that ARSE ≤7.5mm was the optimal cut-off value to predict reduced exercise intolerance in HFpEF patients (AUC=0.91; p<0.001).
Conclusion: We found that, patients with HFpEF have reduced ARSE, which was significantly associated reduced 6MWTD. Reduced ARSE was correlated with subclinical LV systolic dysfunction and diastolic dysfunction. We suggest that ARSE, as a simple echocardiographic parameter might be of value, in order to better discriminate HFpEF patients risk profile.
Keywords: aortic root, heart failure, subclinical LV dysfunction; STE, speckle tracking echocardiography, LVGCS, left ventricular global circumferential strain
HFpEF, heart failure with preserved ejection fraction; ARSE, aortic root systolic excursion
Heart failure with preserved ejection fraction (HFpEF) is a major public health problem, accounting for 50% of HF admissions,1 with comparable mortality and morbidity to heart failure with reduced ejection fraction.2 HFpEF is a heterogeneous disease, with multiple probable mechanisms underlying the clinical syndrome.3
Definitely, HFpEF still considered a medical challenge, as there is no complete understanding of its pathogenesis. Furthermore, in HFpEF subjects, several reports identified various echocardiographic predictors of medical importation,4,5 quality of lifetime,6 and exercise tolerance.7
Anatomically, the root of aorta is a conduit between the LV and ascending aorta. Anterior and posterior walls of aorta move anterior during systolic phase and posterior during diastolic phase.8
Several studies investigated the association between aortic root systolic excursion and left ventricular systolic functional indices. These studies reported that ARSE may represent an echocardiographic marker for left ventricular systolic, especially when Global strain is not achievable or apical views are not available. Furthermore, aortic root has high echogenicy and ARSE can easily be imagined by M-mode echocardiography.9,10
However, the ARSE in patients with HFpEF is not clearly investigated. We hypothesized that aortic root systolic excursion (ARSE) as a simple echocardiographic marker, could predict exercise tolerance in subjects with HFpEF. Hence, our aim was to explore the relation of ARSE with exercise tolerance and its association with subclinical systolic dysfunction in HFpEF patients.
Eighty subjects with HFpEF were enrolled for the research (mean age 53.5±11.3 y, 45% of them were female). The study included patients with: (1) typical symptoms of HF; (2) LVEF more than 50%; (3) abnormal LV relaxation, blunted early mitral annular velocity (e'); high E/e' and B-type natriuretic peptide (BNP) >400pg/ml.11,12 They were compared with 80 healthy subjects, who matched with patients in age and sex.
We excluded patients with Acute Myocardial Infarction, unstable angina, pacemaker implantation, dilated left ventricle, cardiomyopathy, and valvular heart problems, atrial fibrillation, chronic obstructive and possible lung disease regardless the type of pulmonary hypertension, liver disorders, chronic kidney disease , thyroid diseases, arthritis, and patients unable to underwent 6 minute walking test. All participates gave an informed written consent and the study was approved by the faculty scientific and ethical committee.
Resting two-dimensional echocardiography
Transthoracic echocardiographic assessment was performed for all subjects with the use of Vivid 9, General Electric Healthcare (GE Vingmed, Norway) equipped with a harmonic M5S variable-frequency (2.5-4MHz) phased-array transducer. All participants were evaluated by the same operator. The obtained parameters included left ventricular ejection fraction (LVEF %), and the left ventricular mass index. Normal left ventricular mass index was considered, when it was 95g/m2 in women and 115g/m2 in men. Left atrium diameter was indexed for body surface area to calculate LA volume index (LAVI ml/m2). Echocardiographic systolic pulmonary artery pressure (SPAP) was calculated as previously described.13,14
Aortic root systolic excursion (ARSE) was assessed by placing M-mode cursor on center of the root of aorta (Figure 1). The amplitude of systolic movement of anterior aortic wall was calculated to represent ARSE (mm). The average of 3 measures were obtained from the far wall in the long parasternal axis view.15
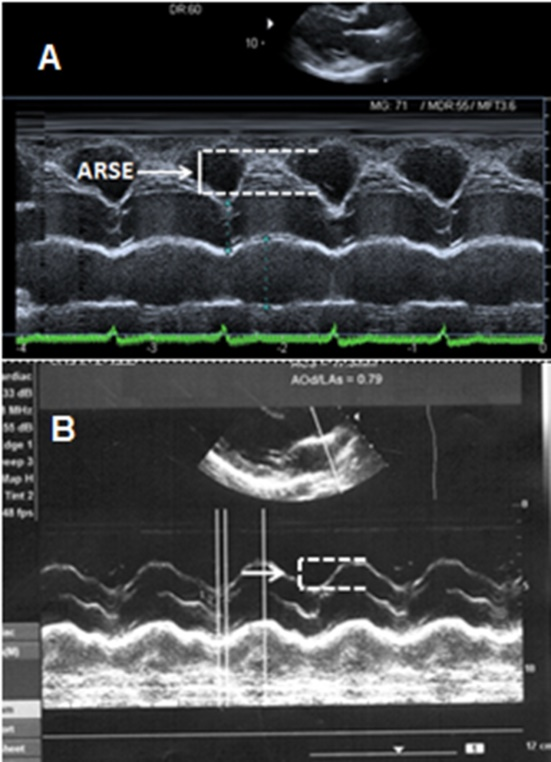
Figure 1 A- Aortic root systolic excursion (ARSE) in patients with good exercise tolerance (6MWTD≥300m). B- ARSE in patients with reduced exercise tolerance (6MWTD<300 m).
Speckle tracking echocardiography (STE)
Two- dimensional gray scale imaging was achieved. Apical two- and four-chamber views were used to assess left ventricular global longitudinal strain (LVGLS). Then we turned to parasternal short-axis views at left ventricular base and mid-level to evaluate left ventricular global circumferential strain (LVGCS). The endocardial border was traced manually at end systole. The entire left ventricular circumference was divided into 6 segments and generated myocardial strain curves by frame-by-frame tracking of the natural acoustic markers throughout the cardiac cycle. With frame rate of 60 to 80frames/s. and at the end of expiratory breath hold, images were acquired and transferred to a workstation for further offline analysis. The offline analysis was performed using automated software On a GE® EchoPAC workstation. Only myocardial segment with good quality by the operator and automatic system were used for analysis.16
Six-minute walking test “6-MWT”
According to Guyatt et al.17 protocol, participates undertook 6MWT. We explained everything with respect to the test for all participates and they were informed to take their drugs. Subjects inquired to walk without any support as far as they can in fifteen obstacle-free hallway, rotating 180° at end of the hallway throughout allotted six minutes. Participates terminated the test, if they experienced any worrying symptoms or signs. Total walking distance was measured and symbolized in meters.
Statistical analyses
Measurement values are expressed as mean ± SD for continuous variables and were compared with two-tailed Student t test. While, qualitative data were expressed as number and percentage and were analyzed by Chi-square (X2) test. Pearson coefficients analysis was used for correlations between variables. We underwent univariate analysis to recognize predictors of six minute walking test distance. Whilst, we underwent multivariate logistic regression to discriminate independent predictors for 6MWTD. All statistical studies were achieved with the use of commercially available SPSS (Version-21).
Table 1 depicts the demographic characteristics of HFpEF subjects versus healthy subjects. All basic data were similar, except for brain natriuretic peptide (BNP), which was higher (P<0.01) in patients with HFpEF. In addition, six-minute walking test distance (6MWTD) was significantly decreased in HFpEF patients (p<0.001).
|
Variable |
HFpEF patients n = 80 |
Control group n = 80 |
P value |
|
Age, y |
57.9±10.5 |
55.3±9.7 |
0.10 |
|
Men, n(%) |
59(73.8%) |
60(75) |
0.57 |
|
Body mass index(kg/m2) |
27.5±3.1 |
25.8±3.0 |
0.15 |
|
Smokers, n(%) |
32(40) |
35(43.8) |
0.51 |
|
Hypertension, n(%) |
62(77.5) |
-- |
|
|
Diabetes, n(%) |
43(53.8) |
-- |
|
|
Coronary artery disease(%) |
19(23.8%) |
-- |
|
|
Heart rate(beats/min) |
75±7 |
70±8 |
0.09 |
|
Systolic BP(mm Hg) |
135±22 |
130±20 |
0.13 |
|
Diastolic BP(mm Hg) |
82±11 |
78±12 |
0.35 |
|
Blood glucose(mg/dL) |
128±11 |
107±5 |
<0.05 |
|
Total cholesterol(mg/dL) |
208±31 |
191±29 |
0.28 |
|
LDL-C(mg/dL) |
135±23 |
128±25 |
0.14 |
|
HDL-C(mg/dL) |
43±8 |
41±7 |
0.62 |
|
Triglycerides(mg/dL) |
158±39 |
139±41 |
0.11 |
|
Creatinine |
1.2±0.4 |
0.95±0.21 |
0.22 |
|
Brain natriuretic peptide(pg/mL) |
208.6±41.5 |
35.8±12.15 |
<.0.01 |
|
6 MWT distance, m |
395±32 |
638±41 |
<0.001 |
Table 1 Characteristics of patients with heart failure with preserved ejection fraction versus controls
LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; 6 MWTD, Six minute walking test distance
With respect to echocardiographic data, Table 2 revealed that, patients with HFpEF had abnormal LV diastolic function [lower e' (P<0.001); increased E/e' ratio (P<0.001) and increased LAVI (P<0.001)] compared with controls. Furthermore, ARSE considerably decreased (P<0.001) in HFpEF subjects than healthy subjects. As far as STE findings, the results showed that both LVGLS and LGCS decreased in the HFpEF patients (P<0.01 and <0.03 singly).
|
|
HFpEF patients n=80 |
Control group n = 80 |
P value |
|
LA volume index(mL/m2) |
35.9±4.8 |
21.5±3.7 |
<0.003 |
|
LV mass index(g/m2) |
96.6±17.5 |
77.3±15.2 |
0.13 |
|
LV EF%(%) |
61.9±3.5 |
64.5±5.3 |
0.29 |
|
E wave |
81.3±4.7 |
61.3±2.3 |
0.17 |
|
S' wave |
6.82±1.30 |
6.91±1.35 |
0.25 |
|
e' wave |
7.23±0.21 |
11.58±0.25 |
<0.01 |
|
E/e′ ratio |
12.5±4.3 |
5.1±1.7 |
<0.001 |
|
SPAP, mmHg |
43±14 |
21±6 |
<0.01 |
|
ARSE, mm |
9.52±1.80 |
12.65±2.21 |
0.01 |
|
LVGLS(%) |
−17.65±2.95 |
−21.15±3.74 |
<0.01 |
|
LVGLSR-s(s−1) |
−1.31±0.19 |
−1.53±0.19 |
<0.05 |
|
LVGCS(%) |
−16.09±4.10 |
−19.32±3.45 |
<0.03 |
|
LVGCSR-s(s−1) |
−1.35±0.18 |
−1.62±0.21 |
<0.05 |
Table 2 Echocardiographic parameters in HFpEF patients versus control subjects
LAVI, Left atrial volue index; LV, left ventricle; LVEF, left ventricular ejection fraction; LVGLS, Left ventricular global longitudinal strain; LVGLSR, Left ventricular global longitudinal strain rate; LVGCSR, Left ventricular global circumferential strain rate; LVGCSR, Left ventricular global circumferential strain rate
We categorized patients with HFpEF into two groups according to the results of 6MWT distance; group with reduced exercise tolerance, included 36 (45%) subjects with six MWT distance <300m and group with good exercise tolerance, included 44(55%) subject with six MWT distance ≥300m. Table 3 represents the baseline data of HFpEF groups. Patients with reduced 6MWTD had increased heart rate (P<0.05), higher systolic blood pressure (P<0.03), high BNP (P<0.03). Furthermore, reduced exercise tolerance group has 6MWTD 256±19, versus 531±41 of group with good exercise tolerance.
|
Variable |
HFpEF with 6MWTD<300 m n = 36 |
HFpEF with 6MWTD≥300 m n = 44 |
P value |
|
Age, y |
59.3±11.5 |
56.8±10.5 |
0.09 |
|
Men, n(%) |
28(77.8%) |
31(70.5) |
0.25 |
|
Body mass index(kg/m2) |
27.3±3.0 |
26.9±3.2 |
0.27 |
|
Smokers, n(%) |
16(44.4) |
18(40.9) |
0.65 |
|
Hypertension, n(%) |
29(77.5) |
33(75) |
0.74 |
|
Diabetes, n(%) |
20(53.8) |
23(52.3) |
0.81 |
|
Coronary artery disease(%) |
9(25%) |
10(22.7) |
0.57 |
|
Heart rate(beats/min) |
88±13 |
73±8 |
<0.05 |
|
Systolic BP(mm Hg) |
139±22 |
127± 19 |
<0.03 |
|
Diastolic BP(mm Hg) |
82±11 |
81 |
0.35 |
|
Blood glucose(mg/dL) |
128±11 |
125±13 |
0.36 |
|
Total cholesterol(mg/dL) |
210±35 |
202±41 |
0.46 |
|
LDL-C(mg/dL) |
138±26 |
132±21 |
0.37 |
|
HDL-C(mg/dL) |
42±7 |
45±6 |
0.52 |
|
Triglycerides(mg/dL) |
163±38 |
157±40 |
34 |
|
Creatinine |
1.19±0.45 |
1.08±0.31 |
0.31 |
|
Brain natriuretic peptide(pg/mL) |
295.6±49.5 |
104.5±24.2 |
<0.03 |
|
Diuretic, n(%) |
25(69.4) |
31(40.5) |
0.58 |
|
ACE-I, n(%) |
21(58.3) |
25(58.8) |
0.98 |
|
ARB, n(%) |
10(27.8) |
11(25) |
0.75 |
|
Beta-blocker, n(%) |
15(41.1) |
19(43.2) |
0.61 |
|
CCB, n(%) |
5(13.8) |
5(11.4) |
0.45 |
|
Nitrates, n(%) |
5(13.8) |
7(15.9) |
0.52 |
|
6 MWT distance, m |
256±19 |
531±41 |
<0.001 |
Table 3 Characteristics of patients with HFpEF with 6MWTD <300m versus those with 6MWTD ≥300 m. preserved ejection fraction versus controls
LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; ACE-Is, angiotensin convertase enzyme inhibitors; ARBs, Angiotensin receptor blockers; CCB, calcium channel blockers
Table 4 depicts the echocardiographic characteristics of patients with 6MWT <300m versus subjects with six MWT distance ≥300m. Patients with reduced exercise tolerance had decreased e' wave (P<0.01), increased LAVI (p<0.01), high E/e' (P<0.001) versus HFpEF patients with 6MWTD≥300m. As regards the LVGLS and LVGCS, we found that HFpEF patients with 6MWTD <300m had lower values than those with 6MWTD≥300m (P<0.001). Figure 2 showed that HFpEF patients had significant decrease in ARSE compared with patients with 6MWTD≥300m and control subjects.
|
|
HFpEF with 6MWTD≥300 m n = 44 |
HFpEF with 6MWTD<300 m n = 36 |
P value |
|
LA volume index(mL/m2) |
32.5±3.3 |
39.8±5.7 |
<0.01 |
|
LV mass index(g/m2) |
95.8±16.5 |
97.5±19.5 |
0.63 |
|
LV EF%(%) |
62.9±3.3 |
61.2±3.1 |
0.49 |
|
E wave |
71.5±3.3 |
89.2±5.1 |
0.26 |
|
S' wave |
6.85±1.2 |
6.79±1.2 |
0.22 |
|
e' wave |
7.92±0.20 |
6.15±0.11 |
<0.01 |
|
E/e′ ratio |
9.1±3.5 |
16.5±4.7 |
<0.001 |
|
SPAP; mmHg |
47±13 |
41±11 |
0.10 |
|
ARSE; mm |
11.54±2.13 |
7.21±1.52 |
<0.001 |
|
LVGLS(%) |
−19.93±3.55 |
−15.83±2.57 |
<0.001 |
|
LVGLSR-s(s−1) |
−1.49±0.17 |
−1.09±0.15 |
<0.03 |
|
LVGCS(%) |
−18.95±4.61 |
−13.01±3.05 |
<0.001 |
|
LVGCSR-s(s−1) |
−1.55±0.22 |
−1.07±0.16 |
<0.01 |
|
HDL-C(mg/dL) |
42±7 |
45±6 |
0.52 |
|
Triglycerides(mg/dL) |
163±38 |
157±40 |
34 |
|
Creatinine |
1.19±0.45 |
1.08±0.31 |
0.31 |
|
Brain natriuretic peptide(pg/mL) |
295.6±49.5 |
104.5±24.2 |
<0.03 |
|
Diuretic, n(%) |
25(69.4) |
31(40.5) |
0.58 |
|
ACE-I, n(%) |
21(58.3) |
25(58.8) |
0.98 |
|
ARB, n(%) |
10(27.8) |
11(25) |
0.75 |
|
Beta-blocker, n(%) |
15(41.1) |
19(43.2) |
0.61 |
|
CCB, n(%) |
5(13.8) |
5(11.4) |
0.45 |
|
Nitrates, n(%) |
5(13.8) |
7(15.9) |
0.52 |
|
6 MWT distance, m |
256±19 |
531±41 |
<0.001 |
Table 4 Echocardiographic parameters in HFpEF patients according to the results of 6 MWT
LAVI, left atrial volue index; LV, left ventricle; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; LVGLSR, Left ventricular global longitudinal strain rate; LVGCSR, Left ventricular global circumferential strain rate; LVGCSR, Left ventricular global circumferential strain rate

Figure 2 The box graph that shows aortic root systolic excursion in patients with HFpEF and 6MWTD<300m compared with those with 6MWTD≥300m and control subjects.
Our results showed a significant correlation of 6MWTD with ARSE (P<0.001), LVGLS% (P<0.001), and LVGCS% (P<0.001). Contrarily, it was inversely associated with E/e' (P<0.02) (Table 5). Likewise, using ARSE as a dependent factor, the correlation analysis (Table 6) revealed that ARSE had strong relation with LVGLS (r=0.59; P<0.001), whilst, it modestly correlated with E/e' and LAVI and LVGCS (P<0.01).
|
Variable |
r |
P value |
P value |
|
LV mass index(g/m2.7) |
−0.13 |
0.19 |
<0.01 |
|
LV EF(%) |
0.17 |
0.11 |
0.63 |
|
Aortic root systolic excursion |
0.62 |
<0.001 |
0.49 |
|
LAVI; ml/m2 |
−0.42 |
< 0.01 |
0.26 |
|
e’ wave |
0.25 |
0.13 |
0.22 |
|
S' wave |
0.27 |
0.11 |
<0.01 |
|
E/e' |
−0.35 |
< 0.01 |
<0.001 |
|
LVGLS(%) |
0.61 |
<0.001 |
0.10 |
|
LVGCS(%) |
0.52 |
<0.01 |
<0.001 |
|
LVGLS(%) |
−19.93±3.55 |
−15.83±2.57 |
<0.001 |
|
LVGLSR-s(s−1) |
−1.49±0.17 |
−1.09±0.15 |
<0.03 |
|
LVGCS(%) |
−18.95±4.61 |
−13.01±3.05 |
<0.001 |
|
LVGCSR-s(s−1) |
−1.55±0.22 |
−1.07±0.16 |
<0.01 |
|
HDL-C(mg/dL) |
42±7 |
45±6 |
0.52 |
|
Triglycerides(mg/dL) |
163±38 |
157±40 |
34 |
|
Creatinine |
1.19±0.45 |
1.08±0.31 |
0.31 |
|
Brain natriuretic peptide(pg/mL) |
295.6±49.5 |
104.5±24.2 |
<0.03 |
|
Diuretic, n(%) |
25(69.4) |
31(40.5) |
0.58 |
|
ACE-I, n(%) |
21(58.3) |
25(58.8) |
0.98 |
|
ARB, n(%) |
10(27.8) |
11(25) |
0.75 |
|
Beta-blocker, n(%) |
15(41.1) |
19(43.2) |
0.61 |
|
CCB, n(%) |
5(13.8) |
5(11.4) |
0.45 |
|
Nitrates, n(%) |
5(13.8) |
7(15.9) |
0.52 |
|
6 MWT distance, m |
256±19 |
531±41 |
<0.001 |
Table 5 Correlation analysis between 6MWD and different echocardiographic parameters in HFpEF patients
LVEF, Left ventricular ejection fraction; LAVI, left atrial volume index; LVGLS, Left ventricular global longitudinal strain; LVGCS, Global circumferential strain rate
|
Variable |
r |
P value |
|
LV mass index(g/m2.7) |
0.11 |
0.25 |
|
LV EF(%) |
0.21 |
0.06 |
|
LAVI; ml/m2 |
−0.43 |
< 0.02 |
|
e’ wave |
0.25 |
<0.05 |
|
S' wave |
0.25 |
>0.05 |
|
E/e' |
−0.39 |
< 0.02 |
|
LVGLS(%) |
0.59 |
<0.001 |
|
LVGCS(%) |
0.46 |
<0.01 |
Table 6 Correlation between ARSE and echocardiographic parameters in HFpEF patients
LVEF, left ventricular ejection fraction; LAVI, left atrial volume index; LVGLS, left ventricular global longitudinal strain; LVGCS, Global circumferential strain rate
Multivariate analysis revealed that ARSE and LVGLS and LVGCS were observed to be significant independent predictors of decreased exercise capacity in patients with HFpEF (Table 7).
|
|
Univariate |
Multivariate |
||
|
Variable |
OR(95%C.I) |
p value |
OR(95%C.I) |
p value |
|
LVEF% |
1.05(0.99–1.08) |
0.41 |
-- |
-- |
|
ARSE |
1.73(1.13–2.41) |
<0.001 |
1.92(1.13–2.71) |
<0.001 |
|
LAVI; ml/m2 |
1.06(1.01–2.98) |
<0.03 |
1.09(0.98–1.75) |
0.21 |
|
E/e' |
1.38(0.95-1.75) |
<0.05 |
0.83(0.95-1.19) |
0.15 |
|
LVGLS |
1.83(0.80-2.93) |
<0.001 |
1.75(0.78-2.71) |
<0.001 |
|
LVGCS |
1.65(0.83-2.45) |
<0.003 |
1.43(0.81-2.15) |
<0.01 |
Table 7 Univariate and multivariate echo predictors for reduced exercise capacity in patients with heart failure with preserved ejection fraction
LVEF, left ventricular ejection fraction; ARSE, aortic root systolic excursion; LAVI, left atrial volume index; LVGLS, left ventricular global longitudinal strain; LVGCS, global circumferential strain rate
ROC analysis was done to detect the best cut-off point to predict exercise intolerance in subjects with heart failure with preserve ejection fraction. Results showed the ARSE≤7.5mm was best cutoff value for predicting exercise intolerance (Figure 3). AUC=0.91, with sensitivity of 93%, specificity of 96%.
The present research revealed: (1) 45% of patients with HFpEF had 6MWTD<300m, (2) HFpEF patient with 6MWTD <300m had lower ARSE as compared with those with 6MWTD ≥300m, (3) significant correlations between ARSE and LV global longitudinal and circumferential systolic strain, whilst ARSE was inversely associated with LAVI, E/e' & BNP, (4) ARSE was an independent predictor of reduced exercise tolerance, and (5) importantly, ARSE≤ 7.5mm was the optimal value for predicting reduced exercise capacity in subjects with HFpEF.
Importantly, our findings revealed that LVGLS and LVGCS in HFpEF patients was lower than control subjects and the decrease was pronounced in subjects with exercise intolerance, implies that HFpEF subjects reduced exercise tolerance had significant subclinical systolic dysfunction. This might be related with presence of more progressive disease process with subclinical systolic dysfunction. Also, straight mechanical ventricular–arterial link affords another justification for the detected association between ARSE and left ventricular systolic performance.
Assessment of systolic displacement of aorta is an earliest deed of echocardiography and spot the starting imaging of functional evaluation of the heart. Nevertheless, principal mechanisms are not fully understood. Aurich M, et al.18 assumed, that aortic systolic excursion might be largely produced by movement of base to the apex of the heart and subsequently could be considered a marker of longitudinal function of left ventricle. Moreover, they supposed that ARSE would be related mainly to left ventricular systolic function. Nevertheless, our findings demonstrated that ARSE also, was associated with E/e' and LAVI. There is no clear underlying pathogenesis, could explain this relationship. However, higher left ventricular filling pressure resulted an increase in LA wall stress and stiffening (structural remodeling of LA) and accordingly, lessens emptying, augment wall stress with decrease compliance and increases BNP secretion.19,20
The reduced ARSE in HFpEF patients with reduced exercise tolerance could be explained by the presence of subclinical systolic dysfunction that could be unmasked with exercise, as our results revealed that ARSE was significantly associated with LV global longitudinal and circumferential systolic strain. Moreover, severe diastolic dysfunction may relate to more progressive disease process, as fibrotic changes, endothelial dysfunction and consequently more impaired contraction and systolic dysfunction.
Aortic recoil possibly, may affect the resistance to the empty of left atrium, reduction pressure, and consequently, aid atrial-ventricular Pressure gradient in physiological conditions. Moreover, recoil of aorta during diastole, following systolic stretch, could ease left ventricular filling and ejections.21 Nevertheless, stiffening of aorta and decreasing its systolic excursion, the valuable influence of stretching and aortic wall recoil to the initial ventricular filling during diastole might be lessened.22
Pratt et al.23 investigated the posterior aortic wall motion displacement with cardiac hemodynamics and established strong relation with left ventricular stroke volume, and they concluded that ASRE is a reaction to the entire left ventricular performance. Moreover, the beginning of ARSE matches with pressure increase and current velocity in the ascending aorta and therefore imitates hemodynamic changes due to contraction of left ventricle.24 Furthermore, it was suggested that posterior aortic wall displacement is mainly influenced by volume changes of LA.25,26
Importantly, the current research showed a negative association between ARSE and both E/e' & BNP level in HFpEF. A finding that provides the value of ARSE in predicting not only, exercise intolerance but also the severity of diastolic dysfunction as well as sbclinical left ventricular systolic dysfunction in HFpEF. A previous study suggested that variations in left ventricular and aortic physiology provide a significant impact in disposition to HF, including HFpEF. This study revealed a significant correlation between stiff aorta and left ventricular global longitudinal performance.27 Furthermore, Kaess et al. assumed that left ventricular diastolic dysfunction as well as more stiffening of aorta might play a significant role left ventricular global longitudinal performance.29
First, single center study with small sample number. Second, the aortic root evaluation motion parameters were obtained utilizing two-D imaging of the PLAX plane. Aortic root movement pathway achieved from parasternal short axis view, where the aortic root is clearly visible. Third, we don't use the 3D motion. The 3D motion information can also be obtained by tracking the aortic root directly on volumetric data available in 3D echocardiography. Finally, intra-observer or inter-observer variability testing was not performed. Finally, most patients in our cohort had pulmonary hypertension in comparison to the control; it would be expected that those population would have exercise intolerance. This compromises the external validity of the study.
We have found that reduced ARSE was significantly associated decreased 6MWTD in patients with HFpEF. These findings concurrently linked to high left ventricular filling pressure, subclinical left ventricular systolic dysfunction and higher values of brain natriuretic peptide. Hence, we suggest that ARSE might be a simple echocardiographic parameter in predicting exercise capacity in cardiac patients, who have normal ejection fraction.
All authors declared that they have no conflict of interest.
None.
None.
None.

©2021 Mahfouz, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.