Journal of
eISSN: 2373-4396


The process of aging and its effect on the histological appearance of the conduction system of the heart have been scantly described. It is not easy to attribute a certain condition to the aging process because it is often impossible to separate physiological aging from the development of pathological changes due to a concomitant disease. Nevertheless, the fact is that atrial fibrillation (AF) is a common arrhythmia in the elderly, and its incidence rise sharply with age. The increase in prevalence of AF in older persons has been reported to be associated with degeneration of the atrial muscle in pathological studies. Evidence in the human atrial muscle of age-related electrical uncoupling of the side-to-side connections between bundles, related to the proliferation of extensive collagenous tissue septa in intracellular spaces was clearly demonstrated. This translates into electrophysiological changes, hence prolonged and fractionated atrial electrograms are recorded from these areas during sinus rhythm by endocardial mapping of the atrium. These abnormal atrial electrograms are characteristics of paroxysmal AF and were found in a significantly greater proportion in older patients than in younger ones with AF. Advancing age is associated with atrial remodeling characterized by anatomical and structural changes, reductions in atrial voltage with discrete areas of fractionated and prolonged atrial endocardial electrograms, widespread conduction slowing as well as anatomically determined functional conduction delay and block. These electrophysiological changes within the atrial myocardium may be responsible substrate for the increased propensity to develop AF with increasing age.
Keywords:aging, atrial myocardium, atrial fibrillation, electrophysiological changes
RI, right inferior pulmonary vein; RS, right superior pulmonary vein; LI, left inferior pulmonary vein; LS, left superior pulmonary vein; LCV, left common vein
Recent developments in diagnostic methodology and novel therapeutic management lead to early recognition and treatment of cardiovascular risk factors and diseases which decreases mortality and increases the proportion of elderly population. Hence, by the year 2035, nearly 25% of the population worldwide will be over 65years of age.1‒4 It is very difficult to attribute a certain condition to the aging process because it is often impossible to separate physiological aging from the development of pathological changes due to a comorbid disease.5‒7 Nevertheless, the fact is that atrial fibrillation (AF) is a common arrhythmia, and its incidence rise sharply with age. Degenerative changes of the atrial myocardium observed in pathological studies were reported to be associated with an increased incidence of AF in the elderly.6‒8
The enhancement of calcium loading predisposes aged atrial cells to develop arrhythmias. Previous canine studies have shown that L-type Ca2+ current is reduced in aged canine right atrial cells compared with adults.9,10 It was shown that the action potential duration in right atrial cardiomyocytes is prolonged with age, and its plateau becomes increasingly negative with age.11‒13 Therefore, there have been described age-related changes at the tissue, cellular, and molecular level of the atrium.
Since detailed human electrophysiological studies describing the influence of increasing age on atrial myocardium properties and remodeling are scanty, it was felt important to investigate and characterize the pattern of atrial muscle changes and remodeling seen with human aging.
Physiopathological changes of the atrium with increasing age
Changes of the conduction system of the heart were previously reported in aged patients without organic heart disease.14‒17 In pathological studies, it was shown a significant loss of myocardial cells with advancing age, and an increase in fibrous tissue within the sinus node and the right atrium.18‒21 These changes are a continuous process which starts in the sixth decade.20 There is a strong association between atrial fibrillation and increased diameter and volume of the atrium. There is an increased incidence of left ventricular hypertrophy in ultrasound studies done in aging population. It was also reported dilation of the left atrium due to left ventricular diastolic dysfunction which was associated to the development of AF.15
Some investigators have demonstrated a significant loss of atrial myocardial cells within the right atrium between the sinus node and the atrioventricular node. It was also shown an increase in fibrosis with loss of myocardial fiber continuity (Figure 1).18‒25 Davies & Pomerance reported that AF in some aging patients was associated with a significant decrease in myocardial cells within the atrium in the absence of any clear pathological cause.18 With this histological and clinical background in mind, changes in the electrophysiologic properties of the human atrium with advancing age can be anticipated to be observed. Since aging has a profound effect on structural changes of the atrium, and since fractionated atrial endocardial electrograms may reflect nonsynchronised, delayed local electrical activity through a diseased atrial muscle,22 it seems reasonable to assume that there may be detectable age induced changes in the electrophysiological properties of the atrial muscle. Therefore, several atrial electrophysiologic parameters may be altered with advancing age. The conduction velocity and ERP are the determinants in the genesis of reentry arrhythmias according to the wavelength theory.23 Wavelength is calculated by multiplying refractory period by conduction velocity, and is definable as the distance traveled by the depolarization wave during the duration of the refractory period.24 If the atrial wavelength is long, reentry may not be maintained and AF may terminate spontaneously.

Figure 1 Four histological cross-sections with trichrome staining.
(A) Gap in the myocardial sleeve composed of fibrous tissue (arrow) extending between the adventitia and a media layer of smooth muscle next to endothelium.
(B) Inter pulmonary myocardial connection at the venoatrial junction (broken arrow).
(C) Inter pulmonary myocardial connection at the ipsilateral anterior wall of adjacent right pulmonary veins (broken arrow).
(D) A focus of fiber disarray and interstitial fibrosis (asterisks) between the left atrium and left superior pulmonary veins.
RI: Right Inferior Pulmonary Vein; RS: Right Superior Pulmonary Vein; LI: Left Inferior Pulmonary Vein; LS: Left Superior Pulmonary Vein; LCV: Left Common Vein. Reprinted with permission from Sanchez-Quintana D, et al. (2012) Triggers and anatomical substrates in the génesis and perpetuations of atrial fibrillation. Curr Cardiol Rev 8: 310-326.
On the other hand, if the atrial wavelength is relatively short because of either a short refractory period, depressed conduction, or both, then a greater number of wave fronts can circulate through the atrium and AF may be sustained. Thus, prolonged atrial conduction delay and/or shortened effective refractory period could be expected to increase the propensity of AF. The occurrence of AF has been demonstrated to depend on not only arrhythmic factors intrinsic to the atrium but also extrinsic factors including autonomics, thyroid function, and lifestyle. Dispersion and lengthening of atrial refractoriness with aging have been reported.26‒30 It was suggested that aging modifies atrial refractoriness in a nonuniform manner, inducing a progressing increment of dispersion of atrial refractoriness.26 It was reported that aging induces a lengthening of atrial refractoriness only at the high right atrium, but not at the mid and low right atrium. It was also demonstrated a nonuniform anisotropic atrium with advancing age.31,32 Several studies reported the age-related changes in the electrophysiological and electrocardiographic properties of the atrium in patients with no history of AF, and showed that the atrial conduction delay was observed in older healthy subjects by the electrophysiological study and the P-wave signal-averaged electrocardiogram.33 However, there are few reports on the age-related electrophysiological changes of the atrium in patients with histories of AF. We have previously demonstrated that aging affects the electrophysiological properties of the atrial muscle induced by programmed atrial stimulation in the patients without documented episodes of AF.30 On the other hand, the aging effects on atrial electrophysiological properties in patients with paroxysmal AF are unclear.
Age-induced changes in atrial endocardial electrograms
We have previously reported that prolonged and fractionated atrial electrograms recorded during sinus rhythm by endocardial mapping of the right atrium are characteristics of paroxysmal AF (Figure 2).34‒37 Furthermore, we have demonstrated that older patients with paroxysmal AF have a significantly greater abnormality of these atrial endocardial electrograms than do younger ones.37 These abnormal electrograms are believed to indicate areas of altered anatomy and conduction where reentrant arrhythmias are likely to develop. We have found a significantly positive correlation between age and the number of abnormal atrial electrograms in man.37 Patients over 60years of age had a significantly greater mean number of abnormal electrograms than those younger ones. In addition, the longest duration and the maximal number of fragmented deflections of atrial electrograms among the 12 right atrial sites also showed a significantly positive correlation with age. We have found a progressive increment in the extension of the electrophysiologically altered atrial muscle with advancing age in man. A prolonged and fractionated atrial electrogram may reflect desynchronized local electrical activity related to a delayed and nonuniform anisotropic conduction through a diseased atrial muscle.37 Therefore, abnormal electrograms indicate areas of altered anatomy and conduction that are suitable substrates for reentrant arrhythmias.22 Since abnormal atrial electrograms increase with advancing age, these electrophysiologic changes may account for the ease to develop atrial fibrillation in elderly patients.
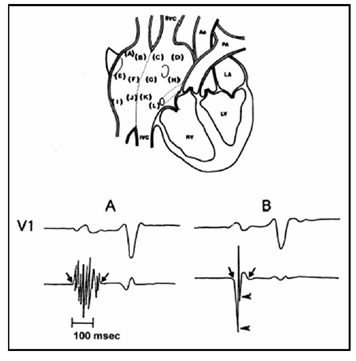
Figure 2 (Top) Twelve endocardial mapping sites in the right atrium. Atrial endocardial electrograms were recorded in each patient from the anterior, lateral, posterior, and medial aspects of the high (a– d), mid (e– h), and low (i–l) right atrium.
(Bottom) (A) Abnormal atrial electrogram with 10 fragmented deflections and 130 ms in duration versus
(B) normal atrial electrogram with 2 fragmented deflections and 80 ms in duration.
Ao: Aorta; IVC: Inferior Vena Cava; LA: Left Atrium; LV: Left Ventricle; PA: Pulmonary Artery; RV: Right Ventricle; SVC: Superior Vena Cava. Reprinted with permission from Centurión OA, et al. (2005) Influence of advancing age on fractionated right atrial endocardial electrograms. Am J Cardiol 96: 239-242.
Kistler et al.,38 observed age-related evidence of generalized conduction slowing in the atria both on standard multipolar electrophysiology catheters deployed along the CS and lateral RA and also during electroanatomic mapping. They also found development of increasing numbers of fractionated signals and double potentials in the atrium but particularly along the crista terminal is with advancing age (Figure 3). This fact may be important because functional conduction delay at this structure has been implicated in the development of a range of atrial arrhythmias. Moreover, electro an atomic voltage maps demonstrated significant reduction in voltage amplitude, which may reflect development of atrial fibrosis (Figure 4).38,39 However, in the absence of histological data, the relationship between decreased atrial voltage amplitud and structural change remains merely speculative. The presence of areas of low voltage has been associated with chronic atrial arrhythmias. Aging was associated with not only a significant reduction in voltage but an increase in the heterogeneity of voltage. In a study in goats with chronic AF, Ausma J et al.,40 observed changes in cellular ultra structure without any alteration in tissue structure. In a subsequent study, they demonstrated that these changes had not completely resolved as late as four months after return to sinus rhythm.41 In another study performed in dogs with atrial remodeling due to chronic heart failure,42 it was reported the persistence of local conduction abnormalities and increased AF duration associated with atrial fibrosis as late as five weeks after resolution of left ventricular dysfunction. Therefore, changes in voltage and conduction seen with aging may represent the structural remodeling and fibrosis, which plays an important role in the development of AF.43‒48
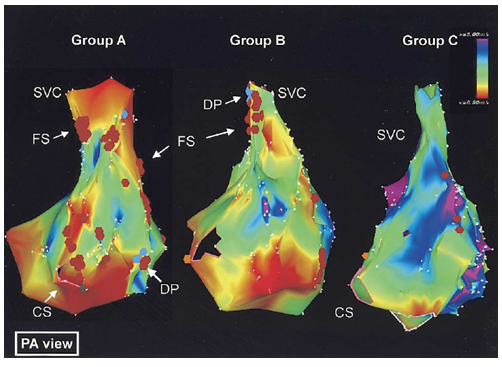
Figure 3 Electroanatomic bipolar voltage mapping.
Color annotation is with bipolar voltages of ≤0.5 mV shown in red, through to voltages ≥5 mV shown in purple. Group A had the oldest patients and Group C the youngest ones, and Group B was intermediate. Electroanatomic activation mapping also demonstrated more extensive areas of double potentials and fractionated signals in groups A and B compared with group C. There was a strong correlation between advancing age and increasing double potentials and fractionated signals.
CS: Coronary Sinus; DP: Double Potentials; FS: Fractionated Signals; SVC: Superior Vena Cava. Reprinted with permission from Kistler PM, et al. (2004) Electrophysiologic and electroanatomic changes in the human atria associated with age. J Am Coll Cardiol 44: 109-116.
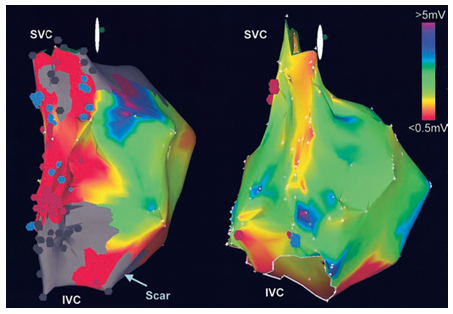
Figure 4 The bipolar voltage mapping is shown in a patient with congestive heart failure (image with the scar) and an age-matched control. Both atria are oriented so that the crista terminalis is en face. The bipolar voltage amplitude in the superior region of the crista terminalis was reduced in patients with heart failure compared with age-matched controls. Note the significant low-voltage regions (red) and scar (gray) along the crista terminalis in the patient with CHF. Blue dots indicate points with DP; brown dots, those with fractionated electrograms. IVC indicates inferior vena cava; SVC, superior vena cava. Reprinted with permission from Sanders P, et al. (2004) Remodeling of sinus node function in patients with congestive heart failure: Reduction in sinus node reserve. Circulation 110: 897-903.
There is increasing knowledge about the precise physio pathological bases for AF initiation and maintenance; however there is still a long way to go in this ever growing field. There has been an overwhelming improvement in technological resources which has lead to a better understanding of the influence of advancing age on the electrophysiological properties of the atrial myocardium in AF patients. Nevertheless, controversies about AF genesis and its relation in the elderly have reemerged.49‒52 Advancing age is associated with atrial remodeling characterized by anatomical and structural substrate changes, reductions in atrial voltage with discrete areas of fractionated and prolonged atrial endocardial electrograms, widespread conduction slowing as well as anatomically determined functional conduction delay and block.53‒57 These changes may be responsible for the increased propensity to develop AF with increasing age in AF patients.
None.
Author declares there are no conflicts of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.