Journal of
eISSN: 2373-4396


The definition of sudden death had to be explained in many other chapters of this book. So I will not go into more details regarding it. The sudden death in the absence of structural heart disease is uncommon, but when it appears, has a very significant clinical impact because many of the victims are young. In relatively recent form it has given special attention to particular conditions expressed in repolarization electrocardiographies on the QT interval, whose relationship with the functionality of some ion channels in the membrane cell has prompted its designation as "channelopathies". But the QT-interval alteration is not unique.
Keywords: electrical cardiac systole disturbances, long and short qt-syndromes, the short pr-interval disturbances, sudden cardiac death
ECG, electrocardiography; WPW, wolff-parkinson-white; TSH, thyroid-stimulating; LBBB, left bundle branch block bundles
Disorders in the electrical cardiac systole can be very dangerous for sudden death, as some cardiac entities previously cited in this book. Its role in sudden death is very important. Before anything else, it is essential to know what is considered as “electrical cardiac systole”. For some authors it would be from the beginning of the Q-wave until the end of T-wave. In contrast, for other authors, including us, it would be from the beginning of the P-wave until the end of T-wave, including the P- wave and the PR- interval (Figure 1). The P- wave, representing atrial activation, the PR- interval is the time from the beginning of atrial activation until the beginning of ventricular activation. The QRS complex represents the ventricular activation. The ST segment and the T- wave represent ventricular repolarization. The QT-interval is the duration of ventricular activation and its recovery. The U-wave probably represents post-depolarization in the ventricles (Figure 2).
Different areas of the PR- interval. ADA = high right atrium. EHH = His bundle interval electrogram. PA = From the high right atrium to the beginning of the P wave in the ECG to the first fast deflection of the lower right atrium; this represents the right intra-atrial conduction, its normal value ranges are between 30 and 50 ms. AH = From the first fast ECG atrial deflection under (A) to the His bundle deflection (H), this represents the intranodal conduction and its normal value ranges are between 45 and 100 ms. The HV-interval value, distance between the His bundle and ventricular muscle its normal length is between 35 and 55 ms (Table 1). It comprises from the beginning of the ventricular complex (Q wave) to the end of the T wave. It measures approximately 0.36 s on average in normal people with a normal heart rate. While overall the QT-interval should be less than half of the interval between two successive R- waves, this rule applies only when the heart rate is normal. Ashmann have established a table that relates the heart rate with the length of the QT interval (QTc corrected) (Table 2).
P-wave. Less than 0.12 sec. in length |
PR-interval. 0.12 sec. to 0.20 sec. in length |
QRS complex. 0.08 sec. to 0.12 sec. in length |
QT-Interval. * 0.40 Sec. to 0.44 Sec. in Length |
Table 1 Standard Values in Length for Waves and Intervals in an ECG Tracing
50 0,414- 0,425 sec |
60 0,386- 0,396 sec |
70 0,361- 0,371 sec |
80 0,342- 0,352 sec |
90 0,321- 0,330 sec |
100 0,297- 0,305 sec |
120 0,283- 0,291 sec |
150 0,252 -0,258 sec |
Table 2 Duration of QT Interval Related to the Frequency Heart Rate (QTc)
(According with Ashmann) The upper limits of these measures should not exceed 10% or more. If so, it would be pathological. However, there are tables for us that are much more useful (Figure 3). These table types are very useful when we trying to find out if a QT-interval is normal, short or long (prolonged) in relation to the heart rate (Table 3).
1º. Short PR-interval Diseases |
2º Cardiac Blockades |
3º QRS-Complex Alterations |
4º Long Qt Syndrome |
5º Short Qt Syndrome |
Table 3 These Table Types are Very Useful when we Trying to Find Out if a QT-Interval is Normal, Short or Long (Prolonged) in Relation to the Heart Rate
Any ECG analysis with a PR- interval value less than 0.12 sec differentiates clinical entities with an early ventricular activation, also called ‘ventricular pre-excitation’, which means that the ventricular activation is produced much earlier than it should be expected when the stimulus is from sinus node, and conduction system is normal. This is so because there are fibers or anomalous conduction pathways that are independent of the normal A-V electrical conduction, connecting the auricles to the ventricles. Since these entities are often associated with paroxysmal tachycardia, is very useful known personal antecedent of episodes of tachycardia and syncope, and also to know if there have fast palpitations with beginning and ending suddenly, which may be a likeness for these pathologies. Currently, we are recognizing four forms of ventricular pre excitation with different electrophysiological substrates. One is quite unknown yet: the clinical entity with a short PR and short QT together on the same ECG tracing (Breijo’s Pattern). These are Table 4.
Wolf-Parkinson-White |
Lown-Ganong-Levine |
Mahaim syndrome |
Breijo’s Pattern |
Table 4 The Clinical Entity with a Short PR and Short QT together on the same ECG Tracing (Breijo’s Pattern)
Wolff-Parkinson-white
Between 1929 -1930, Wolff, Parkinson, and White described a cardiological entity in a series of patients who had a bundle branch block pattern on electrocardiography (ECG); they found, a short PR interval, and paroxysmal tachycardia.1,2 Most of patients were young teenagers. Pre-excitation was defined by Durrer et al.,2 in 1970 with the following statement, Pre-excitation exists, if in relation to atrial events, the whole or some part of the ventricular muscle is activated earlier by the impulse originating from the atrium than would be expected if the impulse reached the ventricles by way of the normal specific conduction system only. While the vast majority of persons with Wolff-Parkinson-White (WPW) syndrome remain asymptomatic throughout their lives, there is a risk of sudden death associated with the syndrome, and is due to very fast tachyarrhythmias (more than 200-250 bpm) that trigger off in the person and that are produced by presence of this accessory pathway.3,4
During episodes of supraventricular tachycardia, the individual may experience palpitations dizziness, difficulty breathing, and sometimes fainting. An ECG displays the typical “delta wave”. The individuals with WPW syndrome have an accessory pathway atrioventricular conduction known as the bundle of Kent. In honour to Kent, who in 1893, found muscle lateral connections in the ring of atrioventricular node, and he thought they were normal atrioventricular connections and however corresponds to an abnormal electrical communication between the atria and ventricles.5 This accessory pathway does not share the properties of AV node, so that electrical activity can lead to a significantly higher rate than the AV node. A rapid heartbeat is potentially dangerous because it can cause hemodynamic instability. In some cases, the combination of an accessory pathway can trigger a ventricular fibrillation, the leading cause of sudden cardiac death. The bundle of Kent is present in a small percentage of the overall population. This pathway is a conduction bundle of tissue that can be found either between the left atrium and left ventricle, in this case as type A pre-excitation or between the right atrium and right ventricle, in this case as type B pre-excitation. The problems arise when this pathway creates electrical circuits that avoid the AV node. The AV node has the property of decreasing the speed of electrical impulses. When performing an abnormal electrical connection through this pathway, tachyarrhythmia is produced.6
In an individual asymptomatic for WPW syndrome this is usually diagnosed by a surface electrocardiogram. It is a typical feature of this syndrome, the delta wave, which is manifested in the slope of the principle of the ascending branch of the R wave, being less pronounced than usual. After a few hundredths of a second, the slope returns to normal. This delta wave, which is verified before and during the initial phase of the R wave carries to a shortening of PR interval and lengthening of the duration of the QRS complex as well as ventricular repolarization disorders. Atrial fibrillation is extremely frequent in symptomatic patients for WPW.6,7 If the patient experiences episodes of atrial fibrillation, the ECG will show a wide QRS complex and a polymorphic tachycardia. This combination of atrial fibrillation and WPW is considered very dangerous.
In individuals with WPW syndrome, the electrical activity that starts in the sinus node travels through the accessory pathway as well as across the atrioventricular node to activate the ventricles through both paths. Since no accessory pathway slows down the electrical impulse (which does make the AV node), this first activates the ventricles through the accessory pathway, and immediately through the AV node. This causes a short PR interval and the presence of delta waves. It is usual that patients with a manifest WPW have often more of an accessory pathway. The treatment depends on the individual risk stratification.8,9 This is performed to determine if individuals with WPW syndrome are at risk for sudden cardiac death (SCD), which in these individuals is due to the propagation of an atrial arrhythmia to the ventricles at a very high rate.
The individuals with WPW syndrome in which delta waves disappear with the increased heart rate are those who have the lowest risk of sudden death.8˗9 This is because the loss of the delta wave proves that the accessory pathway cannot conduct the electrical impulses to a high rate (in the antitrade direction). Generally, these individuals have no fast conduction the accessory pathway during episodes of atrial fibrillation Classic ECG findings that are associated with Wolff-Parkinson-White syndrome include the following Table 5.
Presence of a short PR interval (< 120 ms) |
A wide QRS complex longer than 120 ms with a slurred onset of the QRS wawe form producing a delta wave in the early part of QRS |
Secondary ST-T wave changes |
Table 5 Classic ECG findings that are associated with Wolff-Parkinson-White syndrome include the following
The location of the accessory pathway, in descending order of frequency, is 53%, the left free wall, 36%, posteroseptal, 8%, right free wall, and 3%, anteroseptal. The presence of concealed accessory pathway accounts for approximately 30% of patients with apparent Supra Ventricular Tachycardia referred for EPS. These patients do not have true Wolff-Parkinson-White syndrome because no delta wave is present, but they do have the potential for orthodromic tachycardia. Patients with mitral valve prolapse can have an association with WPW, but the mechanism is unclear. Wolff-Parkinson-White syndrome is found in persons of all ages but most patients with Wolff-Parkinson-White syndrome present during childhood. However, a second peak of presentation is noted in school-aged children and in teenagers. This interesting bimodal age-distribution is due to permanent or transitory loss of pre-excitation during childhood in some patients and during youth. In asymptomatic patients, antegrade conduction across the accessory pathway may spontaneously disappear with advancing age.
Once identified and appropriately treated, WPW syndrome is associated with an excellent prognosis, including the potential for permanent cure through catheter ablation. Asymptomatic patients with only pre-excitation on ECG generally have a very good prognosis. Patients with a family history of Sudden Cardiac Death or significant symptoms of tachyarrhythmias have worse prognoses. However, even in patients with asymptomatic WPW, the risk of Sudden Cardiac Death is increased above that of the general population. Some studies hypothesized that 2 mechanisms are involved in the pathogenesis of AF in patients with Wolff-Parkinson-White syndrome: one is related to the abnormal pathway that predisposes the atria to fibrillation, and the other is independent from the abnormal pathway and is related to increased atrial vulnerability present in these individuals.4,5 According to the literature, risk factors for the development of AF in the setting of WPW syndrome include advancing age (2 peak ages for AF occurrence are recognized, one at 30years and the other at 50years), male gender, and prior history of syncope (Figure 4 & 5).
Electrophysiological image:
A Wolff-Parkinson-White during sinus rhythm. Negative delta wave in V1 and positive in V5, with a short PR-interval. On the right, an episode of atrial fibrillation with high pre-excitation in the same leads, symptomatic for syncope and need immediate electrical cardioversion. The first three rows correspond to the DII electrocardiographic leads V1 and V5, and the following four intracavitary proximal AV junction, AV junction distal ablation catheter and right ventricle (Figure 6). In many more occasions that we can think, Wolff-Parkinson-White can go together to other cardiac electrical disease.10 The cryptogenic Wolff-Parkinson -White is not uncommon. We will put two graphic examples.
Wolff-parkinson-white alongside a long Qt interval10
(Breijo-Marquez FR, Pardo Ríos M (2011) Wolff-Parkinson-White and Prolonged “Q-T” Patterns in the Same Electrocardiographic Record. J Clinic Experiment Cardiol 2:118. doi:10.4172/2155-9880.1000118.) (Figure 7).
Electro-physiology Study. In the EPS graphic can be seen (arrow) typical signs of a WPW: Intracardiac electrogram showing under right ventricular pacing, atrial activation precocity in distal coronary sinus followed by His bundle (Figure 8-10).
Lown-ganong-levine
This syndrome was described in 1952 by Lown, Ganong, and Levine; it is considered a pre-excitation syndrome.11 Currently two types are the more known as pre-excitation syndromes:
This last is characterized by the association of a short PR interval (in adults, less than 0.12sec) and various types of supraventricular tachyarrhythmias (regular tachycardia, atrial flutter, and atrial fibrillation). Etiology• acquired forms. • Congenital form: - Inheritable. - Not inheritable. The familial form is inherited as an autosomal dominant feature and has been associated with the gene encoding PRKAG2 AMP activated protein kinase, responsible for transporting and preserving the heart energy. A mutation in this gene could explain the susceptibility of the heart to the crises of tachycardia. Has been identified a mutation on the long arm of chromosome 7 (7q34-q36). Several structural abnormalities have been proposed as the possible base for Lown-Ganong-Levine, including the presence of James fibers, Mahaim fibers, and fibers of Brechenmacher et al. 12 as well as an underdeveloped sinus node (hypoplastic). Each of these fibers can only be identified histologically. Thus, unless other studies can show structural or functional abnormalities, the definitive diagnosis of Lown-Ganong-Levine remains a clinical diagnosis. In the absence of significant structural heart disease, the mortality rate appears to be very low.13,14 Many people can debut with an acute episode of tachycardia or a history of symptoms suggestive of paroxysmal tachycardia. For its diagnosis needs to be done at least:
The diagnosis of Lown-Ganong-Levine syndrome, based on ECG interpretation:
Its anatomical substrate can be given by:
There is an accelerated nodal conduction when three conditions are met:
In patients with nodal conduction accelerated, the nodal re-entry shows not significant differences in the cycle compared with patients whose PR interval is normal. This is because the tachycardia cycle is primarily determined by the speed from slow pathway conduction. Instead, may increase the incidence of supraventricular tachycardia, because the fast pathway has better drivability. But, this clinical entity has a small role for trigger off a sudden death.
Differential diagnosis with wolf-parkinson-white:
Despite apparently very similar, there are differences, in our opinion, which are critical with respect to drug treatment of choice. The main differences are:
Mahaim syndrome
Between 1938 and 1947 Mahaim and colleagues described a series of anatomical connections between the atrioventricular node and interventricular septum and between the fascicles of normal conduction system and ventricular myocardium.18 It has been demonstrated that most patients with antidromic atrium-ventricular tachycardia with morphology of left bundle branch block bundles (LBBB) have the anatomical substrate in atriofascicular rights bundles.19˗24 It is not a frequent cause for paroxysmal atrioventricular tachycardia by re-entry and it is due to an accessory pathway as Mahaim fibers. These Mahaim fibers have anterograde conduction, with decreased properties in its proximal portion, and they have not conduction capability retrograde (Figure 12). The related tachycardia has a typical left bungle branch block type QRS, due to pre-excitation of the right ventricle through this pathway, closing the rentry circuit through the right bundle branch, His bundle, atrioventricular node and right atrium.21,23
In 1988, in patients undergoing surgery for tachyarrhythmias, was identified this type of accessory pathway, by means of studying its histological substrate. The ventricular insertion is presumed to be in the same branch right, in its distal portion, or apex ventricular myocardium. Since then, the surgical process with catheter- ablation of Mahaim tachycardia has allowed us knows that tachycardia is conducted on the atrium right or on right ventricle free wall.24,25 The resulting tachycardia, to stimulate the ventricles through this beam anomalous (Mahaim fibers) , give us an image QRS with similar aspect of a left bundle branch block, closing the circuit to the atria by retrograde conduction through the right bundle, His bundle and atrioventricular node. With the development of surgery and radiofrequency ablation of supraventricular tachyarrhythmias has been determined this kind of Mahaim conduction is due, in most cases, to paths located in the right free of septum, which cross the ring tricuspid, joining the right atrium with the distal portion of the right branch of the His bundle.24,25 Typical antidromic tachycardia with left bundle branch block image. In leads II and III, negative P waves can be observed immediately after the QRS complex (RP short, long PR) (Figure 13 & 14).
Breijo’s pattern
In 2006, Breijo-Marquez & Pardo Ríos et al.,10,26 evaluated a series of young patients, who had had, since childhood, many episodes of nocturnal palpitations, chest pain, full loss of consciousness (syncope), and which were accompanied by tonic-clonic seizures. All had been diagnosed and treated as epileptic episodes. Treatment outcomes were null. They were always considered as normal in every cardiac studies performed absolutely. However, all these patients had an ECG recording common: A PR-interval lesser than 0,120 seconds with a QTc-interval equal or lesser than 0.350seconds. That is, a pattern of short PR and QTc in the same person. The correct treatment was begun (beta-blockers and, in some cases, an implantable cardio defibrillator (ICD.). Was removed all treatment from epilepsy. The outcome to date is satisfactory. We do not know yet the exact etiology for this pattern: there are not positive genetic studies for a short QT-interval. That is, is negative for these genetic mutations of short QT types. This can say us that we are in front of a possible and little known syndrome new. ECG to date, we know that there were two important misperceptions:
This ECG recording may be easily confused with a Lown-Ganong-Levine, since both have a short PR-interval. Nevertheless, in this type of ECG pattern there is also a short QTc interval. Unfortunately, both entities are confused with epileptic episodes often. Most important clinical features are: Crises always occur at night during rest. The more frequent onset hours are between three and six in the morning.
It is by definition an emergency since of not taking treatment in time, a sudden death is almost certain. Unfortunately this cardiological entity is more frequent than that in principle may seem. It is an almost unknown entity (Figure 15 & 16). This ECG recording was the first with 12 leads that was obtained from our Hospital in Boston. MA. The patient was a 37years-old male. We can see a shortening of the PR and QT intervals (Bazett), especially in inferior and left precordial leads. PR-interval length is lesser than 0.120seconds and QTc length is lesser than 0.350seconds. Patient had the symptoms exposed previously. He was also diagnosed for epileptic episodes. However, he had syncopal episodes by cardiological disturbances: a short PR-interval alongside a short QTc (Figure 17 & 18), (Table 6).
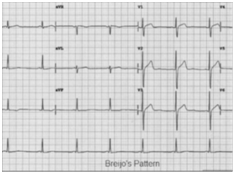
Figure 15 Typical ECG Image of the “Short PR-Interval Alongside a Short QT-Interval” Intervals in the Same Person (during “Vigil Period”): Breijo’s Pattern.
Entity |
PR-Interval |
QRS Complex |
QTc-Interval |
W.P.W |
Short. |
Wide (δ- wave) |
Normal |
L.G.L |
Short |
Normal |
Normal |
Mahaim |
Normal or short |
Normal or wide |
Normal |
Breijo’s Pattern |
Short |
Normal |
Short |
Table 6 Differences among these Syndromes
The interruption of electrical conduction in the heart leads to what has been called: heart blocks.27˗29 They can occur anywhere in the conduction system. So, when atrial activation wave (P wave) takes more than 0.20 seconds to activate the AV node, we speak of first-degree AV block. This blockage, if isolated, can be considered as normal (particularly in elderly people). But there are already many studies linking its presence with increased episodes of atrial fibrillation and the need for a pacemaker. There may also be an electrical outage from the AV node to the Bundle of His. When this phenomenon occurs intermittently, we say that there is a second-degree AV block. We can consider that there are three types of second-degree block: When an atrial activation (P wave) does not reach the ventricles, the QRS complex does not occur. This event is called Mobitz type II.28 There may be a progressive elongation of the PR interval until the stimulation wave cannot reach the ventricles and therefore, is not followed by a QRS complex. This already has been prior described as Wenckebach Phenomenon. When atrial stimulation cannot reach the ventricles, the ventricular activation comes from some ventricular centres, with a slower ventricular rate. We speak of Complete AV block. This type of blockage is very dangerous and is indispensable pacemaker implantation. These three types of block, along with left bundle branch block, are the most dangerous at the time of, being able to produce sudden death. All other are called, blocks of bundle of His in its two branches, which may be Complete or Incomplete. At first, have not importance for Life. Excepting, the left bundle branch block of the His bundle.
Normally, the electrical impulse travels down both the right and left branches at the same speed. Thus, both ventricles contract at the same time. Occasionally there’s a block in one of the branches, so impulses must travel to the affected side by a detour that slows them down. That means one ventricle contracts a fraction of a second slower than the other. Usually if there’s nothing else wrong, a person with bundle branch block shows no symptoms. But a bundle branch block shows up as an abnormality when the electrical impulses through the heart are recorded with an electrocardiogram (ECG). Usually no treatment is required, but your healthcare provider will want to see you regularly to be sure no other changes occur. It is important to have regular check-ups.
Summing up
Sometimes the electrical signal from the heart’s upper chambers (atria) to lower chambers (ventricles) is impaired or doesn’t transmit. There are several degrees of heart block. First-degree heart block occurs when the electrical impulse moves through the AV node more slowly than normal. The heart beat usually has a slower rate and may not cause noticeable symptoms such as light headedness and dizziness and may not require specific treatment. Certain drugs can cause first-degree heart block. These drugs include digitalis, beta-blockers, and calcium-channel blockers. Digitalis is one common drug that is used to slow down the heart rate. If it’s taken in large amounts or for a long period of time, digitalis can cause first-degree heart block. Beta-blockers inhibit the part of the nervous system that speeds up the heart. This increases the conduction delay of the heart’s electrical impulse and can cause first-degree heart block. Among its other effects, calcium-channel blockers have the ability to slow down the conduction of the AV node, resulting in heart block. People with this type of block along with regular assessment by a physician, should also be taught to take their pulse and monitor it regularly to detect changes.
Second-degree heart block occurs when some electrical signals from the atria do not reach the ventricles, resulting in “dropped beats.” Second degree heart block can be classified as Mobitz Type 1 or Mobitz Type 2. Mobitz Type 1 is also commonly referred to as Wenckebach and may not cause noticeable symptoms. Symptoms associated with second degree heart block are chest pain, faintness (syncope), and palpitations, breathing difficulties, such as shortness of breath with exertion, rapid breathing, nausea, and fatigue. Second degree Type I may not require treatment but can be a forerunner for Type 2 and needs to be monitored on a regular basis as well as daily pulse checks by the patient. However, it can be a forerunner for Mobitz Type 2 and needs to be monitored by a physician.
In Mobitz Type 2, the heart beat does not beat effectively and impacts the hearts’ ability to pump blood to the rest of the body. Often times having a pacemaker inserted is necessary so the heart will beat effectively. Third-degree or complete heart block means that the heart’s electrical impulse does not pass from the heart’s upper to lower chambers at all. When this occurs, the heart does not beat correctly and cannot effectively move blood to the body. Secondary pacemaker cells in the lower chambers will take over, causing the ventricles to contract and pump blood, but at a slower rate than when signals come from the sinoatrial node. Complete heart block in adults is caused by heart conditions or as a side effect of drug toxicity. An injury to the electrical conduction system during heart surgery also may cause heart block. People with third-degree heart block experience irregular and unreliable heart beats, which requires immediate medical attention involving a temporary pacemaker because of the potential for having a cardiac arrest. A permanent pacemaker would be indicated to treat complete heart block (Figure 19).
The QRS complex is one of the components of a typical electrocardiogram (ECG) tracing of a complete cardiac cycle, normally occurring after the P wave (atrial depolarization) and before the T wave (ventricular repolarization). It is a graphical representation of the cardiac impulse travelling into the ventricular myocardium. To further understand its morphological characteristics and clinical relevance, a brief discussion of the QRS complex is provided below. About 120 to 200milliseconds (ms) after the generation of an action potential in the SA node, the cardiac impulse reaches the ventricles through the atrioventricular (AV) nodal/His bundle system. The cardiac impulse is conducted to the ventricular mass through the bundle branches and the Purkinje fibers. Normally, the QRS complex is seen as a “sharp” complex, resulting from the rapid depolarization of the ventricles via the fast conducting Purkinje fibers of the bundle branches. Compared to the intra-atrial conduction speed, the conduction speed of the bundle branches is high, averaging about 4 meters per second. The first deflection of the QRS complex is called a Q wave when it is negatively deflected (pointing downward) and an R wave when it is positively deflected (pointing upward). A positive deflection following a Q wave is called an R wave; and a negative deflection following an R wave is designated as an S wave. A second positive deflection after the R wave is called an R’ (R prime).
A QRS complex may not always contain a Q, an R or a S. Many combinations of these deflections, as well as isolated deflections are generally referred to as QRS complexes. Upper case and lower case letters are used to describe not only the type of waves or deflections present but also the general orientation of the QRS complex. A prolonged duration of the QRS complex signifies a conduction delay in the ventricles. Being an important part of the ECG curve, the QRS complex is routinely analyzed when investigating cardiac pathologies. However, all the components of the ECG must be considered and correlated clinically to ensure an accurate diagnosis.
Since its discovery, much has been written about this cardiological entity. Possibly this entity is the most studied alongside the atrial fibrillation (which, incidentally, should be included in this type of disorder). In 1856, Meissner described the sudden death of a young, deaf girl who was being berated at her school academy. Inadvertently, this was perhaps the first reported description of the Long QT Syndrome. A more detailed clinical characterization of deafness, prolonged QT interval on ECG and propensity to sudden cardiac death followed more than a century later when Jervell and Lange-Nielsen described a series of patients. A similar syndrome, but without deafness, was identified to run in families and was first described by Romano et al. in 1963 and later by Ward in 1964. The clinical spectrum of the Long QT Syndrome is now well characterized, with affected patients vulnerable to recurrent syncope or cardiac arrest from a polymorphic ventricular tachycardia known as torsade de pointes.30 The hypothesis of the relationship between Long QT Syndrome and Sudden Infant Death was studied by Schwartz31 by means of QTc measurements in the first week of life. In your follow-up, 34 children died, 24 of them from Sudden Infant Death.
Arnestad et al. studied 7 genes associated with Long QT Syndrome in 201 cases of sudden infant death, found that 9.5% of infants who died suddenly showed some of the mutations studied and showed that long QT is a cause which contributes significantly to sudden death in infants. Wang et al. studied the association between Sudden Infant Death and the presence of Long QT Syndrome, 5 variants (S216L, T1304M, F1486L, F2004L, and P2006A) had alterations in Na + channels, typical of SCN5A mutations associated with Long QT Syndrome. Authors speculated perform ECG screening programs in newborns, which has generated controversy despite the results obtained by several investigators. Incredible but true: a good management on an EKG study could save thousands of lives in the newborn. And however, there are many controversies yet. The fact that malignant forms of Long QT Syndrome are associated with a marked prolongation of the QTc interval could encourage the conduct of investigations by ECG and genetic screening in infants susceptible to suffer it, which would help not just to consider a good preventive therapy but also identify to all affected members in the same family.30 Long QT Syndrome can happen from mutation of one of several genes.30˗35 These mutations tend to prolong the duration of the ventricular action potential, thus lengthening the QT interval. LQTS can be inherited in an autosomal dominant or in an autosomal recessive manner. The autosomal recessive forms of Long QT Syndrome have a propensity to have a more severe phenotype, with some variants having associated syndactyly (Long QT 8) or congenital neural deafness (Long QT 1).
A number of specific gene loci have been identified that are associated with Long QT Syndrome. Genetic testing for Long QT Syndrome is clinically available and may help to direct appropriate therapies. The most common causes of Long QT Syndrome are mutations in the genes KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3) 32. According to expert consensus, the preventive and definitive treatment for sudden death by channelopathies is the implementation of an automatic implantable defibrillator in all patients with cardiogenic syncope, or sudden cardiac death recovered. But the prospects of new treatments are already being carried out. Conduction damage may cause arrhythmias, per example the Long QT, which are primarily treated pharmacologically or by medical device-based therapies, including defibrillation and tissue ablation. Nevertheless, drug therapies sometimes may not be effective or are associated with serious side effects. Device-based therapies for cardiac arrhythmias, even with well developed technology, still face inadequacies, limitations, hardware complications, and other challenges. Therefore, scientists are actively seeking other alternatives for antiarrhythmic therapy.
In particular, cells and genes used for repairing cardiac conduction damage/defect have been investigated in various studies both in vitro and in vivo. Despite the complexities of the excitation and conduction systems of the heart, cell and gene-based strategies provide novel alternatives for treatment or cure of cardiac arrhythmias in general and of Long QT particularly. In addiction, there are peculiarities in some of them, in Long QT Syndrome are indicated changes in lifestyle, in the case of Long QT Syndrome 1 are contraindicated some sports, especially swimming, football . In the Long QT Syndrome 2, should avoid exposure to acoustic stimuli mainly during sleep. Beta blockers are the first line of treatment in all patients with a diagnosis of Long QT Syndrome, even in asymptomatic patients because it reduces the risk of events up to 60%. Implantation of pacemaker and left sympathetic ganglionic denervation are therapeutic options in Long QT Syndrome. Nevertheless, the current essays on this latter technique are still quite disappointing. There are still many discrepancies between different authors for to know which are the ranges for be considered as long (or prolonged) a QTc. We must also say that the LQT can be produced by external agents (non-genetic) as drugs and some systemic disorders, such as anemia. We believe that every person diagnosed with chronic anemia should be thoroughly assessed from the point of view electrocardiographic, and accurately measure the length of the QT interval.36˗38
It was first described in 2000 in a handful of patients, and since then 3 different genes associated with the disease and the biophysical basis have been described. Gussak, Brugada R. and associates have described for the first time what has now been defined as a clinical syndrome. They identified a short QT interval in three persons from the same family, one of whom had several episodes of paroxysmal atrial fibrillation.33˗39 The definitive link between short QT syndrome and sudden death on a family unit was described by Gaita and associates in 2003. In 2004, the genetic and biophysical bases, for this cardiological entity as well as a possible therapeutic approach were provided.
The short QT syndrome is characterized by a ventricular fibrillation in the absence of structural heart disease with presence of a QTc interval <350msec. In most of the reported cases, there is a history of sudden death in the family and some affected individuals also have atrial fibrillation. The presence of a short QT interval in the ECG is associated with susceptibility to the ventricular arrhythmias, and it is linked to the ventricular refractoriness with the origin of this disorder. Confirmation of a ventricular effective refractory period significantly shortened (<150msec) during programmed ventricular stimulation, supports this concept. As already we have said, this cardiological entity is a channelopathy and, in the particular case of short QT syndrome, it is postulated that the delivery of atrial and ventricular channels IKs, IKr, ICa-L determines a gain of function of potassium channels or the loss of calcium channel function , results in a reduction of atrial refractoriness and vulnerability to the onset of atrial fibrillation (three genes-KCNH2, KCNQ1 and KCNJ2-encoding different potassium ion channels involved in repolarization have been linked to this syndrome). Electrocardiogram. The QTc interval <350msec in the absence of external involved factors, make to suspect the presence of this syndrome. The coexistence of acuminate T wave, alongside a high QRS voltage in precordial leads is not a consistent finding in all the reported cases.40
Electrophysiological Study
During programmed ventricular stimulation from the right ventricle most of the patients had inducible ventricular fibrillation with 2 and 3 extra stimuli. Half of the patients also had atrial fibrillation inducibility. The ventricular effective refractory period was set at values below 150 msec, and the infusion of flecainide and other drugs of I-class determine a prolongation between 30 and 100msec.40,41
Short Qt-Interval (ECG 12 Leads)
Electrocardiogram of short QT syndrome. Observe the tall peaked T waves. (From article “short QT syndrome” by R. Brugada et al. in Journal CMAJ.) (Figure 20).
A prolonged QT interval has long been known to be a harbinger of life-threatening ventricular arrhythmias, and long QT syndrome is recognized as a hereditary condition with an increased risk of sudden cardiac death. A short QT interval, on the other hand, was not considered arrhythmogenic and was seen mostly in relation to hypercalcemia. This way of thinking changed after the first description in 2000 of a sporadic case of short QT interval and sudden cardiac death and of a family with short QT interval and paroxysmal atrial fibrillation. The short QT syndrome constitutes a new clinical entity that is associated with a high incidence of sudden cardiac death, syncope, and/or atrial fibrillation even in young patients and newborns. Patients with this congenital electrical abnormality are characterized by rate-corrected QT intervals lesser than 350 ms. The implantable cardioverter defibrillator is the therapy of choice in patients with syncope and a positive family history of sudden cardiac death. However, ICD therapy in patients with a short QT syndrome has an increased risk for inappropriate shock therapies due to possible T wave oversensing. In patients with a mutation in HERG, it turn into ventricular tachycardias/ventricular fibrillation non-inducible and restored the QT interval/heart rate relationship towards a normal range. It may serve as an adjunct to ICD therapy or as a possible alternative treatment, especially for children and babies (newborns).42
The authors of this chapter also have a considerable number of patients who have a variety of abnormalities in the heart’s electrical systole. Combinations “short-long” “long-short” at different intervals are not uncommon. They need to be more valued. At first they may be overlooked. And however, they are also able to develop a sudden death (Figure 21). The association between structural and electrical challenges is not unusual. The author of this chapter, has highlighted associations such as a pattern of “PR and QT short” (Breijo’s Pattern) with a pattern of Wellens26,37, for example Figure 22.
None.
Author declares there are no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.