Journal of
eISSN: 2373-4396


Background: Assessment of viability could be of significance in ischemic and heart failure patients before deciding for revascularization. The use of Dobutamine stress echocardiography has the disadvantage of subjective visual evaluation of regional wall motion, so, new technologies have been modified; one of these is to assess mitral annular velocity using tissue Doppler imaging.
Aim: The aim of this study was to evaluate the value of Pulsed wave tissue Doppler mitral annulus velocity with dobutamine echocardiography in assessment of myocardial viability and prediction of functional recovery of wall motion abnormalities after revasularization in patients with coronary artery disease.
Patients and methods: The study group included 40 patients, selected consecutively from patients presented to Ain Shams university Hospitals with coronary artery disease as proved by diagnostic coronary angiography and recommended for potential coronary revascularization. Each patient underwent baseline transthoracic echocardiography, in addition to low dose dobutamine echocardiography using TDI at mitral annulus in 6 different walls. All patients were subjected to revascularization (30 by PCI and 10 by CABG) then followed up after 6 months by transthoracic echocardiography to assess improvement in EF and SWMA.
Results: Using the 16 segment method, a total of 640 segments were studied: 250 Segments were considered nondysfunctional (39%), 390 Segments were dysfunctional: of which, severely hypokinetc (216, 55.4 % of abnormal), 158 segments akinetic (40.5% of abnormal), and 16 Segments were dyskinetic (4.1% of abnormal). The mean SWM index at rest was 1.9 (0.39).Using low dose dobutamine echocardiography, 220 segments were detected to be viable (56.4%), while 170 were non-viable (43.6%). In order to relate the results of TDI, The 16 segments were reevaluated into 6 walls per patient. Using this method, 240 walls were studied. 19 walls were excluded due to technical difficulties in assessment of TDI. Pulsed wave TDI demonstrated that dysfunctional areas had lower systolic velocities compared to areas considered as normal. Similarly, the increase from baseline to DSE (ΔTDI) was higher in nondysfunctional areas vs. dysfunctional areas. However, there was no significant difference in the mean TDI at rest for viable and non-viable walls as detected by dobutamine stress echocardiography. But the increase in TDI velocity with peak dobutamine was significantly more in viable (1.97±0.44) vs. non-viable (1.14±0.54) walls, with p<0.0001.The ejection fraction improved from 39.87±8.22 (mean + STD) at baseline to 46.13±8.58 (mean + STD) at follow-up. The score index of the segmental wall motion during follow up after revascularization was 1.45±0.29 (mean+STD). Among the total dysfunctional segments detected at baseline echocardiography, follow-up echo postoperatively showed improvement by ≥1 score in 239 segments (61.3 %).In the 220 dobutamine positive segments, 182 segments improved postrevacsularization (82.7%) while 38 did not show improvement (17.3%), whereas in the segments designated as dobutamine non-viable, 122 segments were not improved (71.8%) and 48 segments (28.2%) improved post revascularization. Taking improvement as the gold standard for viability, sensitivity of dobutamine is: 82.7%, specificity 71.8%, PPV 79.1%, NPV 76.3%. Using TDI method, 115 (79.3%) walls were diagnosed as viable by this method, while 30 walls were nonviable (20.7%). 80 walls (55.2% of total studied walls) were detected to be viable by both dobutamine conventional 2D echocardiography and dobutamine TDI echocardiography (systolic wave), On the other hand, 27 walls (18.6% of total studied walls) were detected to be non-viable by both methods. However, 38 walls (26.2% of total abnormal walls) showed discordance between the two methods. The improvement of 74 walls of 80 combined TDI& DSE positive walls, making a sensitivity of 90.2%, similarly 24 of 27 concordant TDI & DSE negative walls did not improve, making a specificity of 92.3%.
Conclusion: The current study confirmed the importance of using TDI in different mitral annular sites, as an objective tool in detecting myocardial viability, and to improve the sensitivity and specificity of DSE.
Keywords: myocardial viability, myocardial hibernation, ischemic cardiomyopathy, revascularization tissue doppler imaging, mitral annular velocity, dobutamine stress echocardiography
SPECT, single-photon emission computed tomography; CAD, coronary artery disease; EF, ejection fraction; TDI, tissue doppler imaging; LVESD, left ventricular end systolic diameter; WMSI, wall motion score index; LVEDD, left ventricular end diastolic diameter; CABG, coronary artery bypass grafting; WMSI, wall motion score index
Coronary artery disease (CAD) remains a principal cause of morbidity and mortality worldwide.1 Many subjects with heart failure and underlying CAD have an important amount of viable but dysfunctional myocardium that may restore the ability to contract normally if perfusion improves.2 This reawakening of myocardium after restoration of blood flow was referred to as "hibernating".3˗5
There are two main theories to explain the pathophysiology of hibetrnation. The first view of adaptation involves dedifferentiation or embryonic regression, the so called “smart heart” hypothesis,6 with a down-regulation in energy utilization and an upregulation of stress proteins.7 This counterbalances the effects of ischemia but at the cost of an attenuated level of contractile function.8,9 The alternative theory is that this is "forced degeneration", supported by the finding that hibernating myocardium also contains apoptotic cells and cells with autophagosomes, lysosomes, and vacuoles.10 Whatever the cause, structural remodeling would be essential to restore contractility, thus chronically impaired but viable myocardium may take weeks or months to recover once flow is restored.11 Interventions that bring back blood flow to the hibernating myocardium may return the myocytes to their physiologic function and reprogram the cells to normal expression of key proteins.12
The differentiation of viable from nonviable myocardium is therefore highly relevant in patients who are being considered for revascularization 13. Many patients who demonstrate viability associated with severe LV dysfunction may still be candidates for revascularization rather than for cardiac transplantation.14
Several Imaging techniques are used to detect viable myocardium, depending on different characteristics of dysfunctional but viable myocardium. The most widely used and available methods are:15,16 Nuclear imaging by Single-photon emission computed tomography (SPECT) (evaluating perfusion, cell membrane integrity, and intact mitochondria with thallium or technetium-labeled agents) and Echocardiography with dobutamine (to assess contractile reserve).
In a recent meta-analysis, all available studies of regional left ventricular function in patients with ischemic left ventricular dysfunction before and after revascularization were pooled.17 This analysis confirmed and extended the findings of the previous pooled analysis by the same group.18 In general, the nuclear imaging techniques had a higher sensitivity and lower specificity than DSE. Regarding prediction of global function improvement, DSE appeared to have the higher specificity, but the differences between techniques were not statistically significant.17
Generally, the final endpoint in viability studies is the long-term prognosis. Several studies and metaanalyses have evaluated the prognostic value of viability in relation to therapy. These data consistently showed better prognosis in patients who had viable myocardium and were revascularized, suggesting that revascularization stabilizes the unstable substrate of dysfunctional but viable myocardium.17,19
It should be noted, however, that medical therapy was not standardized in the studies analyzed by Allman K et al.,19 and the adherence to optimal therapy was not adequately described. In the last decade, the medical treatment of heart failure has continued to improve and significant advances have been made in the techniques for coronary revascularization which have reduced intra-procedural and peri-procedural risks.20
Consequently, Camici P et al.,21 pooled the data from 14 non randomized studies found a trend for a survival benefit in patients with CAD and LV dysfunction, with viable myocardium, who underwent revascularization compared with patients with viable myocardium treated medically. In the absence of viable myocardium, no clear-cut difference can be observed between treatments despite the fact that advances in both modalities of coronary revascularization procedures have reduced intra-procedural and peri-procedural risks. Most of these studies were based on retrospective analysis. On the contrary, reviewing the most recent literature, it was observed that the annual mortality rate in patients treated medically appears to be similar regardless of the presence of viability.22
In a substudy from STICH trial22 601 patients with CAD and LV dysfunction were enrolled in a randomized trial of medical therapy with or without CABG, using SPECT, DSE, or both to assess myocardial viability. The study concluded that although the presence of viable myocardium was associated with a greater probability of survival in patients with CAD and LV dysfunction, however the assessment of myocardial viability did not recognize patients who can benefit from CABG, as compared with medical therapy alone. This finding may reflect the low rates of death among patients with viable myocardium who received medical therapy alone in STICH study (~7% per year), as compared with previously reported rates.22 Recently, Gerber et al studied 144 patients with coronary artery disease and myocardial dysfunction and concluded that detection of functional viable myocardium by DE-CMR is an independent predictor of mortality in patients with ischemic LV dysfunction before revascularization. This conclusion may be useful for pre-operative selection of patients for revascularization .23
Echocardiography can allow detection of myocardial viability with a rather reasonable accuracy, using pharmacological stress echocardiography.24 In patients with jeopardized but viable myocardium, the LV ejection fraction (EF) will show improvement with low-dose dobutamine in direct proportion to the number of segments with contractile reserve.25 Dobutamine-induced segmental and global functional recovery correlates well with other, more complex imaging techniques, including PET and thallium scintigraphy.26,27 Furthermore, new developments in stress echocardiography can help as adjuvant to improve viability detection. These include contrast echocardiography, tissue Doppler imaging (TDI) and strain, and three-dimensional echocardiography.
The use of TDI for viability prediction at rest has been limited by its lack of site specificity because the segment of interest can be “tethered” by neighboring segments. Some TDI parameters including peak systolic velocity, isovolumetric contraction, and time-to-peak systolic velocity have not been shown to consistently predict functional recovery.28,29
Myocardial velocity analysis by TDI at rest and during dobutamine stimulation could allow assessment of myocardial viability.30˗32 Pulsed Doppler tissue velocity analysis has been performed on apical views with analysis of systolic tissue velocities confined to the basal segments. This approach allows assessment of viability for a whole ventricular wall from apex to base.33 Pulsed wave TDI has the ability to quantify myocardial wall motion velocities. Several factors can influence TDI measurements, such as the translational and rotational motion of the heart within the thorax, as well as the angle of incidence of the ultrasound beam relative to the axis of myocardial longitudinal movement. By assessing myocardial velocities from the apical views, the effect of translation and rotation of the heart on the measurement of myocardial velocities is minimized. In addition, the apex acts as a fixed reference point facilitating the assessment of contraction and relaxation in the axial plain without the need for angle correction. A possible limitation in the evaluation of TDI results may be tethering between adjacent regions that can influence its ability to localize differences in myocardial velocity. To avoid this effect, tissue Doppler tracings were obtained with the sample positioned near the mitral annulus to assess the vectorial sum of contraction velocities of the longitudinally oriented myocardial fibres between the base and the apex.30
The aim of this study was to evaluate the value of Pulsed wave tissue Doppler mitral annulus velocity with dobutamine echocardiography in assessment of myocardial viability and prediction of functional recovery of wall motion abnormalities after revasularization in patients with coronary artery disease.
Our study group was selected consecutively from patients presented with coronary artery disease as proved by diagnostic coronary angiography and recommended for potential coronary revascularization. Inclusion criteria included the presence of significant (>50%) reduction in the luminal diameter of a major coronary artery on the basis of recent coronary angiographic results in addition to regional left ventricular wall motion abnormality on the basis of two-dimensional echocardiography. Patients with decompensated heart failure, significant valvular heart disease, technically difficult echocardiography, or with contraindication to dobutamine administration were excluded from the study.
The purpose of this study was explained to all participants and informed consent was taken, approved by ethical committee.
In addition to history taking, physical examination, and ECG, all patients were subjected to the following: Baseline two–dimensional transthoracic echocardiography was performed for all patients. Images were acquired with the patient in the left lateral decubitus position using a 2.5MHz transducer attached to a commercially available VINGMED VIVID VI machine equipped with a harmonic imaging capability. The machine was used to measure the left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), Ejection fraction (EF), and resting segmental wall motion. For analytical purposes, the left ventricle was divided into the standard 16-segment model recommended by the American Society of Echocardiography.34 A wall motion score index (WMSI) serving as an index for the extent of dysfunction was derived by dividing the sum of individual segment scores by the number of interpretable segments.34 A WMSI of 1.00 indicated normal wall motion.
Dobutamine stress echo test
An intravenous access was secured for eligible patients, three bipolar ECG leads were connected to the patient's chest for continuous monitoring throughout the study and Dobutamine infusion was used in a stepwise manner (resting, 5 and 10ug/kg/min) during 3minutes intervals. At each dose heart rate and blood pressure were measured.
Standard echocardiographic views (Parasternal long axis, parasternal short axis, apical four chamber view, apical two chamber view) were recorded with the patient in left lateral decubitus at baseline and at peak infusion. Visual analysis of wall motion and thickening was performed using the previously described scoring system and standard 16-segment model recommended by the American Society of Echocardiography. Regional wall motion score improvement by ³ 1 grade in at least two contiguous nonoverlap segments at any stage of pharmacologic stress infusion compared with the baseline study indicated viability.35,36
End points for interrupting the infusion protocols were
Dobutamine stress mitral valve annulus pulsed tissue Doppler imaging
The echocardiography machine was switched to TDI mode to encode myocardial velocities. The systolic mitral annular motion velocities at 6 mitral annular sites were detected as follows: (anteroseptal and inferolateral walls in the apical long axis view, inferoseptal and anterolateral walls in the apical 4 chamber view, and anterior and inferior walls in the apical 2 chamber view). The peak systolic wave was determined at each of these walls, both at rest and at peak dobutamine infusion. The acoustic power and filter frequencies of the ultrasound scan system were set to the lowest values possible and the sample columns (width of approximately 8 mm) was set at the mitral annulus.37
Revascularization procedure and followup
The infarct related epicardial coronary artery stenosis was revascularized by either coronary artery bypass grafting (CABG) or by percutaneous transluminal coronary intervention (PCI). Follow up transthoracic two dimensional echocardiogragraphy and TDI was done after 6 months of the revascularization to detect improvement in regional wall motion and the left ventricular function compared to the basal resting echocardiographic study.
The results were tabulated and statistically analyzed. Specificity and Sensitivity of dobutamine mitral annular tissue Doppler imaging (TDI) AND dobutamine echo were calculated and compared against the gold standard of viability (improvement with revascularization).
Categorical variables will be expressed as number (%) and continuous variables will be expressed as mean ± SD. The independent sample t-test and ANOVA will be used to compare the mean values of different groups. Linear regression will be used for correlation analyses, which were expressed as Pearson correlation coefficients. For all tests, p value < 0.05 will be considered statistically significant. All the analyses will be performed with commercially available software (SPSS version 19.0; SPSS, Inc., Chicago, IL, USA).
The study included 40 patients with documented significant coronary artery disease by coronary angiography and scheduled for elective revascularization, by PCI or CABG. Table 1 & 2 shows basic characteristics, echocardiography and coronary angiography data. Regional wall motion abnormalities (dysfunctional segments) were present in all patients, with a mean ± SD of: 9.75±3.5 dysfunctional segments per patient. Mean hypokinetic segments/patient = 5.4±2.9, akinetic = 3.9±2.3, while mean dyskinetic/patient = 0.4±0.9.
Parameter |
Value |
Age Mean±SD |
49±8.7 |
Males No. (%) |
34 (85 %) |
Smokers No. (%) |
29(72.5%) |
Diabetic No. (%) |
23(57.5%) |
Hypertension No. (%) |
32(80%) |
Positive family history No. (%) |
15(37.5%) |
Overweight No. (%) |
27(57.5%) |
NSTEMI no. (%) |
5(12.5%) |
STEMI no. (%) |
22(55%) |
CHF |
19 (47.5%) |
Baseline Echocardiographic Parameters |
|
LVEDD Mean±SD |
58.9±7.9 |
LVESD Mean±SD |
44.5±8.9 |
IVS Mean±SD |
10.6±2.2 |
PW Mean±SD |
10.7±2 |
EF Mean±SD |
39.9±8.2 |
Table 1 Summarizes the basic demographic and echocardiographic characteristics of the study patients
Angiographic Findings |
Number of Patients |
Percentage |
1 Vessel Disease |
10 |
25% |
2 Vessel Disease |
19 |
47.5% |
3 Vessel Disease |
11 |
27.5% |
Site of lesion |
Number of patients |
Percentage |
LM |
4 |
10% |
LAD |
27 |
67.5% |
LtCx |
29 |
72.5% |
RCA |
23 |
57.5% |
Table 2 Results of Coronary Angiography
Using the 16 segment method, a total of 640 segments were studied: 250 Segments were considered normal or mildly hypokinetc (nondysfunctional) (39 %), 390 Segments were dysfunctional: of which, severely hypokinetc (216, 55.4 % of abnormal and 33.75 % of all), 158 segments akinetic (40.5% of abnormal, 24.7 % of all), and 16 Segments were dyskinetic (4.1% of abnormal, and 2.5 % of all). The mean SWM index at rest was 1.9 (0.39).
Detection of myocardial viability
Detection of viability was done to the 40 patients by using conventional Low dose dobutamine 2D echocardiography and Tissue Doppler at mitral annulus (TDI). The normokinetic segments at rest detected by 2D conventional echocardiography were excluded from the study, so the remaining diseased walls were 390.
The ejection fraction at rest was 39.87±8.22 (mean + SD), while during maximal rate of dobutamine infusion during the study was 45.2 ±8.4 (mean + SD). (P value <0.0001). The score index of the segmental wall motion at rest was 1.9±0.3 (mean + SD), while during maximal rate of dobutamine infusion during the study was 1.55±0.31 (mean + SD). (P value <0.0001).
Among the 390 affected walls, 220 segments were detected to be viable (56.4%), while 170 were non-viable (43.6%). Among the hypokinetic segments, 120 segments were viable by DSE, representing 55.6 % of hypokinetic segments, while 96 segments were viable among the akinetic segments (60.8%) and 4 of the 16 dyskinetic segments were viable, representing 25% of dyskinetic segments (Figure 1). The mean viability index per patient (number of viable segments divided by number of dysfunctional segments) was 0.56.
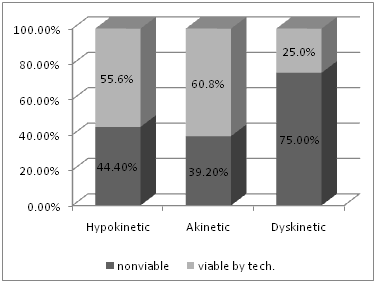
Figure 1 Showing the percentage of viable and nonviable segments in hypokinetic, akinetic and dyskinetic areas, as assessed by DSE.
In order to relate the results of TDI, we reevaluated the 16 segments, where Wall motion was scored by the pattern displayed by two thirds of the sub segments, making 6 walls per patient.32 Using this method, 240 walls were studied. Nineteen walls were excluded due to technical difficulties in assessment of TDI, (2 in anterior, 2 in anteroseptal, 6 inferoseptal, 2 inferoir, 3 anterolateral, 4 inferolateral) this makes the feasibility of these walls 95%, 95%, 85%, 95%, 92.5%, and 90%, respectively. Analysis of the pulsed-wave Doppler tissue sampling velocity profile showed a significant morphological variation for each wall and with dobutamine.
There were 76 walls (31.7%) defined as normal/mild hypokinetic walls; 13 in anterior, 12 in anteroseptal, 12 inferoseptal, 14 inferoir, 10 anterolateral and 15 inferolateral. The remaining 145 walls (60.4%) were assessed by both DSE and TDI systolic velocity: Using DSE, viability was detected in 86 walls (59.3%), while 59 segments were nonviable (40.7%).
There was variability in mean TDI for each wall (Table 3). Pulsed wave TDI demonstrated that dysfunctional areas had lower systolic velocities compared to areas considered as normal (5.86±0.7 vs 7.41±0.76, p < 0.0001). This result was similar in each wall separately as in the following table. Similarly, the increase from baseline to low dose DOB. (ΔTDI), was higher in nondysfunctional areas vs. dysfunctional areas: This was true in all walls (2.49±0.41 for nondysfunctional vs., 1.55±0.63 in dysfunctional areas, p <0.0001) and in each wall separately as in the table.
TDI/Wall |
Mean TDI(SD) |
Mean TDI in Normal Areas (SD) |
Mean TDI in Dysfunctional Areas(SD) |
P Value For Normal and Dysfunctional Areas |
Anterior |
6.34 (1.12) |
7.33 (1.09) |
5.93 (0.86) |
< 0.0001 |
Anteroseptal |
6.39 (1.06) |
7.58 (0.79) |
5.86 (0.58) |
<0.0001 |
Inferoseptal |
6.36 (1.05) |
7.62 (0.77) |
5.87 (0.67) |
<0.0001 |
Inferior |
6.26 (1.1) |
7.34 (0.54) |
5.49 (0.77) |
<0.0001 |
Anterolateral |
6.27 (0.94) |
7.28 (0.83) |
5.87 (0.64) |
<0.0001 |
inferolateral |
6.42 (0.99) |
7.31 (0.55) |
5.95 (0.92) |
<0.0001 |
Table 3 TDI for each wall, in dysfunctional &non-dysfunctional areas
However, There was no significant difference in the mean TDI at rest for viable and non-viable walls as detected by dobutamine stress echocardiography (5.83 ± 0.73 in viable vs. 5.85±0.76 in nonviable walls, with p=0.9 nonsignifcant). But the increase in TDI velocity with peak dobutamine (Δ TDI) was significantly more in viable (1.97±0.44) vs. non-viable (1.14±0.54) walls, with p<0.0001. This was statistically significant for the overall walls as well as for each wall site separately (Figure 2 & Table 4).
TDI/Wall suit |
Normal Areas Mean (SD) |
Dysfunctional Areas |
P Value |
Anterior |
2.32 (0.34) |
1.89 (0.32) |
0.003 |
Anteroseptal |
2.68 (0.38) |
1.83 (0.37) |
<0.0001 |
Inferoseptal |
2.43 (0.34) |
1.87 (0.67) |
0.014 |
Inferior |
2.53 (0.39) |
2.1 (0.27) |
0.002 |
Anterolateral |
2.52 (0.53) |
1.8 (0.32) |
0.003 |
Inferolateral |
2.47 (0.45) |
1.74 (0.77) |
0.015 |
Table 4 Showing TDI change (ΔTDI) in normal and abnormal areas in different walls
Assessment post-revascularization
Thirty patients (75%) underwent PCI, while 10 patients (25%) underwent CABG. No major acute coronary events were detected in the candidates post revascularization during the followup period. At followup, The LV EF at rest was 46.13±8.58 (mean + SD). P value was <0.0001, compared to baseline EF). The score index of the segmental wall motion at rest measured during follow up after revascularization was 1.45±0.29 (mean + STD). P value was <0.0001, compared to baseline SWMI).
Among the total dysfunctional segments detected at baseline echocardiography, follow-up echo postoperatively showed improvement by ≥1 score in 239 segments, representing 61.3 %. Mean No. of segments improved per patient 5.9. On analysis, 126 of 216 hypokinetic segments improved post revascularization (58.3%), while in the 158 akinetic segments, 107 segments improved (67.7%). Six of the 16 dyskinetic segments improved postrevascularization (37.5%).
Value of DSE and TDI in Relation to Post-Revascularization Echocardiography
In the 220 dobutamine positive segments, 182 segments improved postrevacsularization (82.7%) while 38 did not show improvement (17.3%), whereas in the segments designated as dobutamine non-viable, 122 segments were not improved (71.8%) and 48 segments (28.2%) improved post revascularization (Figure 3). Taking improvement as the gold standard for viability, sensitivity of dobutamine is: 82.7%, specificity 71.8%, PPV79.1, NPV 76.3.

Figure 3 Relation between DSE viability and postoperative improvement in all dysfunctional segments.
On analysis of different types of regional wall motion abnormalities; for hypokinetic segments, sensitivity is 82.5, specificity: 71.9, ppv: 78.6, npv: 76.7 For Akinetic segments, sensitivity: 91.7%, specificity 69.4%, PPV: 82.2%, NPV: 84.3%. Finally, for dyskinetic segments, sensitivity 100%, specificity 83.3%, PPV 66.7%, NPV100%.
A cutoff value of ≥ 1 cm/sec in systolic velocity at low dose DSE was used to define viability of dysfunctional segments.32,38 This made 115 (79.3%) walls to be diagnosed as viable by this method, while 30 walls were nonviable (20.7%). Eighty walls (55.2% of total studied walls) were detected to be viable by both dobutamine conventional 2D echocardiography and dobutamine TDI echocardiography, this represents 93% of the total number of walls found to be viable by the dobutamine conventional echocardiography and 71.4% of the walls viable by TDI. On the other hand, 27 walls (18.6% of total studied walls) were detected to be non-viable by both dobutamine conventional 2D echocardiography and dobutamine TDI echocardiography, this represents 45.8 % of the total number of walls discovered to be non-viable by the DSE. However, 38 walls (26.2% of total abnormal walls) showed discordance between the two methods (Table 5 & Figure 4).
|
Positive DSE |
Negative DSE |
Total |
||||
Improved |
Not Improved |
Total |
Improved |
Not Improved |
Total |
||
+ve TDI |
74 |
6 |
80 |
19 |
14 |
33 |
113 |
TDI-ve |
0 |
6 |
6 |
2 |
24 |
26 |
32 |
Total |
74 |
12 |
86 |
21 |
38 |
59 |
145 |
Table 5 Showing concordance and discordance between DSE [six walls] and TDI systolic wave in relation to improvement of dysfunctional segments post revascularization.
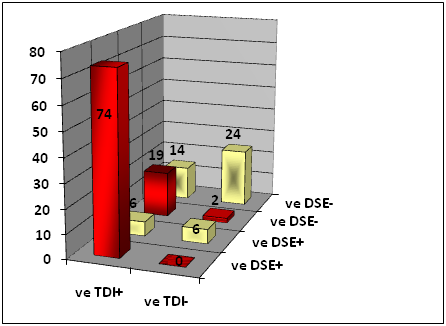
Figure 4 Showing concordance and discordance between DSE [six walls] and TDI systolic wave in relation to improvement of dysfunctional segments post revascularization.
The most significant finding in this table was the improvement of 74 walls of 80 combined TDI& DSE positive walls, making a sensitivity of 90.2%, similarly 24 of 27 concordant TDI & DSE negative walls did not improve, making a specificity of 92.3%.
SWM score/EF improvement
In the present study, the EF at rest was 39.87±8.22 %( mean ± SD), while during maximal rate of dobutamine infusion during the study was 45.2 ±8.4 % (mean ± SD). The score index of the segmental wall motion at rest was 1.9 ±0.39 (mean ±SD), while during maximal rate of dobutamine infusion during the study was 1.55±0.31 (mean ±SD). At follow up, The LV EF at rest was 46.13 ±8.58 (mean + SD). (P < 0.0001). The score index of the segmental wall motion at rest measured during follow up after revascularization was 1.45±0.29 (mean + STD). (P <0.0001).
In other studies, similar results were obtained: Carluccio E et al.,36 showed that low-dose dobutamine infusion elicited a substantial improvement of both EF (from 32±7 to 41±8 %, P <0.0002) and WMSI (from 2.45±0.33 to 1.85±0.36, P < 0.0002). Similarly, In the study by Carluccio E et al.,39 where Patients were reevaluated 7.6 ± 3.3months after revascularization, revascularization significantly improved LV function, ejection fraction increased from 33± 6 % to 45±10 % (p <0.0001) and WMSI from 2.29±0.31 to 1.74 ±0.42 (p <0.0001).
Low Dose DSE
In the present study, low dose dobutamine had sensitivity: 82.7%, specificity 71.8%, PPV 79.1, NPV 76.3 in predicting regional improvement after revascularization.
This is comparable with the meta-analysis by Schinkel A et al.,17 where Subanalysis of the pooled data showed that low-dose dobutamine echocardiography (33 studies, 1121 patients) had a sensitivity and specificity of 79 and 78%, with a PPV and NPV of 76 and 82% in prediction of recovery of regional function 17. However sensitivity is significantly more in our study which may be attributable to different kinds of patients in the different studies involved in the meta-analysis.
In another metanalysis by Camici P et al. 21, low dose dobutamine stress had 76% sensitivity, 81 % specificity, 66 % PPV and 89 % NPV 21. Similarly, comparing the present study with some of the available individual studies; La Canna G et al.,40 Studied 28 patients with coronary artery disease and LV dysfunction (EF≤50), low dose dobutamine, was found to have sensitivity of 74, specificity 74 at 12 months post revascularization.40 Aggeli C et al.,41 in a study on Forty-one patients with coronary artery disease and left ventricular dysfunction (ejection fraction ≤40%), at follow up after revascularization, found low dose dob to have sensitivity 78%, specificity 85%, PPV 87, NPV 76%.41 Tani T et al.,42 investigated the agreement between low-dose dobutamine stress echocardiography and (FDG-PET) and compared each technique’s ability to detect myocardial viability and predict functional recovery in 30 patients who underwent revascularization, followed by echocardiography 5±3 months : Low dose dobutamine echo was found to have sensitivity 84%, specificity 80%, PPV 88%, NPV 75% 42. Karabinos I et al. 43 evaluated the diagnostic accuracy of DSE in detecting myocardial viability in subgroup of 86 patients with known coronary artery disease before undergoing PTCA with stent implantation. DSE was found to have sensitivity 90%, specificity 100%, positive predictive value 100%, and negative predictive value 91% in the detection of myocardial viability.43
The difference in the results between these studies, including our study, may be related to different patients' characteristics, different inclusion and exclusion criteria, different revascularization procedures and different time for follow up echo post revascularization. Also, in the present study, there was a relatively high incidence of akinetic segments (40% of all dysfunctional segments), which may have more structural changes including fibrosis, cellular dedifferentiation, and loss of contractile elements 44 than less dysfunctional myocardium. It is expected that such segments may require longer times for functional recovery following revascularization45,46 and our single postoperative follow up at six months may have underestimated the amount of recoverable LV dysfunction.
Tissue Doppler
Several studies in the past have shown that pulsed wave TDI is an effective method to assess quantitatively the existence of myocardial viability after myocardial infarction.
In our study, Pulsed wave TDI demonstrated that dysfunctional walls had significantly lower systolic velocities compared to walls considered as normal. (5.86±0.7 vs. 7.41 ± 0.76, p < 0.0001). Similarly, the increase from baseline to DSE was higher in non-dysfunctional walls vs. dysfunctional walls: (2.49 ± 0.41 for non-dysfunctional vs., 1.55±0.63 in dysfunctional areas, p <0.0001). However, There was no significant difference in the mean TDI at rest for viable and non-viable walls as detected by dobutamine stress echocardiography (5.83±0.73 in viable vs. 5.85±0.76 in nonviable walls, with p=0.9 non-significant). But the increase in TDI velocity with peak dobutamine was significantly more in viable (1.97±0.44) vs. non-viable (1.14±0.54) walls, with p<0.0001, indicating that these regions were hibernating.
The same findings were found in several studies
Bountioukos M et al.,47 in a study on 93 pt., Pulsed-wave TDI at rest demonstrated that dysfunctional regions had lower systolic velocities compared with non-dysfunctional regions (6.2 ±1.9cm/sec vs. 7.1 ±1.8cm/sec, P <0.001). There was no difference in systolic velocity at rest between viable and nonviable regions (6.3±1.9 cm/sec vs. 6.3±1.9cm/sec, respectively, P < 0.93). However, during low-dose dobutamine infusion, systolic velocity was significantly higher in viable regions (8.5 ±2.7cm/sec vs 7.8 ±2.4cm/sec, respectively, P<0.002).47 In another study by Bountioukos M et al.,47 on 70 pt with ICM,. Myocardial systolic velocity of normal or mildly hypokinetic regions (non-dysfunctional regions) was 6.8± 2.0cm/sec at rest and 9.2± (3.3)cm/sec during low dose dobutamine challenge, while ΔVs was 2.4±2.8cm/sec. Dysfunctional regions had significantly lower velocities: 6.2±1.6cm/sec at rest (p<0.001) and 7.6±2.0 cm/sec at low dose dobutamine (p < 0.001), with ΔVs reaching 1.4±1.5 cm/sec (p <0.001). In dysfunctional regions with contractile reserve during low dose dobutamine challenge, maximum Vs was 6.1 ±1.6 cm/sec at rest and 8.0±2.2 cm/sec during low dose dobutamine, and ΔVs was 1.9 ±2.1 cm/sec. In dysfunctional regions without contractile reserve, Vs was 6.2 ±2.0 cm/sec at rest (p = 0.41) and 7.3±2.2 cm/sec during low dose dobutamine (p = 0.04), and ΔVs was 1.1 ±2.0cm/sec (p = 0.008).30 These results were also found in other studies.32,37,38,48
Cutoff value for viability
In the present study, a cut off value of ≥ 1cm/sec in systolic ejection velocity (Vs) at low dose dobutamine infusion was used to define viability of dysfunctional regions. This value was calculated as the best significant value using ROC method. This resulted in 115 (79.3%) walls to be diagnosed as viable by this method, while 30 walls were nonviable (20.7%).
This cutoff value was used by Bountioukos M et al. 38, and resulted in the detection of 69.1% of all dysfunctional regions as viable. (60% and 73% of Q wave and nonQ wave dysfunctional regions respectively) 38. Altinmakas S et al. 49 also used similar procedure and assuming 35% as a cut-off for viability the increase in Systolic velocities by DSE yielded an 89% sensitivity and 86% specificity for predicting post-revascularization functional recovery.49
Aggeli C et al.,41 Using ROC curves, the optimal cut-off value for viability assessment was an increase of 0.5cm/sec in ejection velocity (S wave) during LDDSE (80% sensitivity and 88% specificity, area under the curve 0.80).41
Rambaldi R et al.,32 used an improvement of velocity of 1±0.5 cm/sec as an index of viability. Pulsed-wave Doppler tissue sampling has a sensitivity of 87%, and a specificity of 52% for the prediction of viable myocardium. This increase exhibited an incremental value to DSE for the diagnosis of myocardial viability. The sensitivity of DSE and pulsed-wave Doppler tissue sampling was 75/87%, respectively (P<0.05), and specificity was 51/52%, respectively (P=ns).32
In the present study, we did not concentrate on the diastolic indices that can be detected by TDI. This is partially because such indices were not consistently shown to be correlated with viability post-revascularization. Rambaldi R et al.,32 found that E/A ratio changes from rest to low dose failed to predict myocardial viability.32 Similarly, Bountiuokos M et al.,30 found no difference between viable and non viable regions with respect to late diastolic velocities at rest and low dose dobutamine. In addition, early diastolic velocities were more age-dependent than systolic velocities.30
The main striking finding of the present study is the improvement of 74 walls of 80 combined TDI & DSE positive walls, making a sensitivity of 90.2%, similarly 24 of 27 concordant TDI & DSE negative walls did not improve, making a specificity of 92.3%. This finding may explained by the amplification effect of basal assessment by pulsed-wave Doppler tissue sampling, which is able to detect even small amounts of viable myocardium disseminated between the base and apex. These findings are consistent with higher myocardial integrity required for an inotropic response than for metabolic uptake.50
Pulsed wave TDI is a feasible and relatively inexpensive technique that can increase the sensitivity of the dobutamine stress test. It appeared to be minimally influenced by loading changes, when compared to standard echo images in case of doubtful viability assessment, due to suboptimal thickness/texture detection.
In many of patients with CAD, the extent of remaining viable tissue is of clinical and prognostic significance. It can help to decide between revascularization and cardiac transplantation. Many subjects with heart failure and underlying coronary artery disease have an important amount of viable but dysfunctional myocardium, where myocardium keeps the ability to contract if perfusion improves. The dysfunctional viable myocardium has unique characteristics which form the basis for the different imaging modalities that are currently available for the assessment of myocardial viability. Recent studies showed that the presence of viable myocardium was associated with a greater likelihood of survival in patients with CAD and LV dysfunction, but the assessment of myocardial viability did not identify patients with survival benefit from CABG, as compared with medical therapy alone. Dobutamine stress echocardiography is the most frequently used agent in this setting to assess jeopardized myocardium for viability. Viability is shown by noting improved contraction of a dysfunctional LV wall segment with low-dose dobutamine infusion, which provides adrenergic stimulation. However, to overcome the subjective visual evaluation of regional wall motion, new technologies have been modified; one of these is to assess mitral annular velocity using tissue Doppler imaging. The current study confirmed the importance of using TDI in different mitral annular sites, as an objective tool in detecting myocardial viability with DSE. Pulsed wave TDI is a feasible and relatively inexpensive technique that can increase the sensitivity of the dobutamine stress test. It appeared to be minimally influenced by loading changes, when compared to standard echo images in case of doubtful viability assessment, due to suboptimal thickness/texture detection.
None.
Author declares there are no conflicts of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.