Journal of
eISSN: 2373-4396


Left ventricular assist devices (LVADs) are increasingly used to treat patients with advanced heart failure both as a Bridge-To-Cardiac Transplantation and Destination Therapy. Device thrombosis is a well-known complication of LVADs that can be potentially catastrophic if not diagnosed early and appropriately managed. We present a case of successful treatment of a suspected Heart Mate II thrombus with systemic thrombolysis and Eptifibatide in a patient who presented with signs and symptoms of acute decompensated heart failure and an ischemic stroke. We further compiled published case reports on acute thrombosis management in contemporary continuous flow LVADs, to discern various treatment approaches available for this serious complication. Published research on optimal anti-coagulation management strategy in the setting of acute device thrombosis is limited, primarily due to the size of this cohort but also due to the nuances involved in tracking such data. We would like to suggest that all potential device thrombosis cases and their management be reported to a registry or added to a existing registry such as INTERMCAS (Interagency Registry for Mechanically Assisted Circulatory Support), as this would not only enhance our understanding on this topic but also serve as a building block for future research and could be used to make evidence based management recommendations.
Keywords: left ventricular assist device, thrombus, thrombolysis, management, recommendations
A 56-year-old Caucasian male with a cardiac history of severe nonischemic cardiomyopathy, 3months post Heart Mate II (Thoratec, Pleasanton, CA) Left ventricular Assist Device (LVAD) implantation, presented to the hospital with a history of acute difficulty in breathing, lethargy and decreased mentation per his wife. He was transferred from an outside hospital about 5 hours since presenting there and during which time he was treated for acute heart failure with intra-venous furosemide. On examination, he was found to be in severe respiratory distress and semi- obtunded. On physical exam he had significant right hemiplegia that was not reported from the outside hospital. A “Stroke Code” was called and a stat- non-contrast-enhanced CT brain was ordered. On connecting his LVAD to the base power module, his parameters were: RPM 10,000, Power-12, Pulsatility Index-2.1, and Flow-3-4 L/min, (Table 1). The higher power and low PI readings in the setting of acute heart failure immediately raised the suspicion of an acute device thrombus. His wife told us that patient’s symptoms of shortness of breath, dizziness and an episode of fall with no loss of consciousness had started about 4 hours prior to presentation at the outside hospital and about 9 hours prior to presentation at our hospital.
|
Day 1- |
Day 3 |
Day 7 |
Day 19 |
Day 29- |
Day 29- |
Day 70- |
|
|
Admission |
Discharged to Rehab |
Discharged to Home |
Current |
||||
|
LVAD RPM |
10,000 |
10,000 |
9800 |
10,000 |
9600 |
9600 |
9600 |
|
LVAD Power |
12 |
7.7 |
7.5 |
7 |
7 |
7 |
6.4 |
|
LVAD Flow |
03-Apr |
6.4 |
6.4 |
6 |
5.6 |
5.8 |
5.3 |
|
LVAD Pulsatility Index |
2.1 |
3.5 |
3.5 |
5.3 |
5.3 |
4.8 |
5.4 |
|
MAP (mmHg) |
64 |
80 |
70 |
68 |
96 |
88 |
78 |
Table 1 LVAD Parameters. LVADs, left ventricular assist devices; RPMs, revolutions per minute
His past medical history was significant for episodes of gastrointestinal bleeds secondary to small bowel AV fistulas, post- mitral valve repair, and right total knee arthroplasty with chronic infection that required long-term Dicloxacillin therapy. His outpatient anti-coagulation regimen was Aspirin 325mg daily and Warfarin with a goal INR of 1.5-2. Due to recurrent episodes of GI bleeds his recent INR goal was between 1.5-2. On presentation patients INR was 1.6. His other medications included Amiodarone 200mg daily, Carvedilol 3.125mg twice daily, Isordil mononitrate 30mg daily, Ferrous Sulfate 324mg twice daily, Bumetanide 1mg daily, Dicloxacillin 500mg twice daily, Allopurinol 300mg daily, Pantoprazole 40mg daily, Tramadol 200mg daily, Hydrocodone-Acetaminophen 10-325 three times daily and Zolpidem 10mg nightly.
Vital signs on presentation at the outside hospital were: Temperature 38.1 F, Heart Rate of 90-101 beats/minute, Respiratory Rate 20-40 breaths/ minute, mean arterial pressure of 70 mmHg and oxygen saturation of 94% on VentiMask 40%. On examination at our hospital he was found to be tachypneic at 33 breaths/minute requiring Bi-level positive airway pressure (BiPAP), heart rate-106 beats/minute, mean arterial pressure-64 mmHg and oxygen saturation of 90% on VentiMask 40%. He appeared confused and lethargic. Neck exam was positive for jugular venous distension. He had decreased breath sounds bilaterally and the LVAD hum was heard on cardiac exam. On neurologic exam, he did not follow any commands, was aphasic with a right facial droop and had right hemiplegia. On checking with the outside hospital, due to his acute distress and lethargy, a neurologic exam was not performed. Laboratory tests on admission were consistent with hemolysis and acute renal failure (Table 2). LDH was elevated at 3012 (IU/L) up from<500 a week ago. Chest radiography was consistent with pulmonary edema (Figure 1). CT Head revealed a left non-hemorrhagic MCA infarct with mild mass effect and mild midline shift from left to right (Figure 2). Urine in his Foley bag was orange- red in color. A stat echocardiogram (Figure 3) obtained revealed a dilated left and right ventricle and an aortic valve opening every beat that was opening every other beat on his last clinic visit, 3 weeks ago. This further confirmed the working diagnosis of an acute LVAD thrombus complicated by an embolic event leading to the acute ischemic infarct. An emergent Neurology consultation was obtained and after reviewing his physical exam and CT-brain with the Neurology team, the diagnosis of acute ischemic stroke with significant right hemiplegia was confirmed. Since he was outside the recommended treatment window for thrombolytic administration for his acute stroke (9hours against the recommended, the Neurology team recommended against the use of systemic thrombolysis due to the increased risk for bleeding vs. reducing infarct size.
|
Day 1- |
Day 3 |
Day 7 |
Day 19 |
Day 29- |
Day 70- |
|
|
Admission |
Discharged to Rehab |
Current |
||||
|
Hemoglobin (g/dl) |
7.6 |
8.8 |
8.8 |
8.4 |
8.7 |
8.9 |
|
Hematocrit (%) |
23.8 |
29.8 |
25.7 |
26.8 |
24.8 |
28.2 |
|
Platelets (K/uL) |
94 |
109 |
111 |
86 |
139 |
204 |
|
LDH (IU/L) |
3012 |
2726 |
2236 |
12126 |
363 |
292 |
|
Plasma free hemoglobin |
||||||
|
Haptoglobin (IU/L) |
<8 |
<8 |
<8 |
<8 |
<8 |
|
|
Total Bilirubin (mg/dl) |
1.8 |
2 |
1.1 |
0.4 |
0.3 |
0.4 |
|
Creatinine (mg/dl) |
1.8 |
1.3 |
1.4 |
0.7 |
1 |
0.9 |
Table 2 Laboratory values
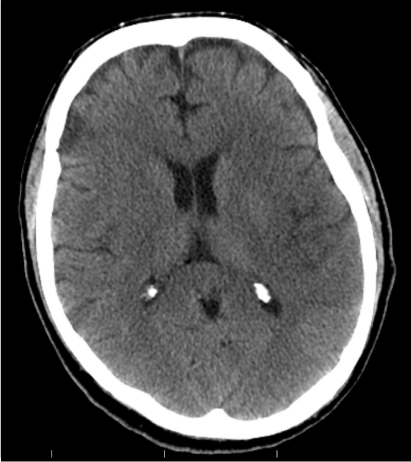
Figure 2 CT- Head at Presentation. Large left non- hemorrhagic middle cerebral artery infarct with mild mass effect.

Figure 3 Echo- at Presentation. Apical-4 chamber view showing a dilated left and right ventricle despite increasing the lvad rpm.
He was administered a recombinant tissue plasminogen activator, Alteplase 70 mg IV X 1 (0.81 mg/kg x 86.2 kg) over 1 minute, followed by 7.7mgs (0.09mg/kg x 86.2kg). His LVAD parameters post-Alteplase infusion were: RPM- 10,000, Power- 7.7, Pulsatility index-3.5, Flow-6.4 L/min. 24-hours post completion of Alteplase infusion; he was started on intra-venous Heparin continuous infusion (--units) with no bolus. CT-Head scans were obtained serially, prior to initiating heparin therapy and during the 1st week and showed evolving ischemic infarct with no intra-cranial hemorrhage (Figure 4). On day 4 with no further decrease in his LVAD power (Table 1) he was started on a continuous infusion of Eptifibatide at 1mcg/kg/min that was increased to 1.5mcg/kg/min for days 6, 7 and continued at 1mg/kg/min for days 8-19. During this time his hemodynamics also improved allowing inotropic support with Milrinone to be weaned off. CT-Head performed on day 16 showed completed ischemic infarct with small stable sub-arachnoid hemorrhage (Figure 5).

Figure 4 Chest- X Ray Post- Thrombolytic Infusion showing
a) Decreased pulmonary vascular congestion
b) Unchanged LVAD cannula position from baseline
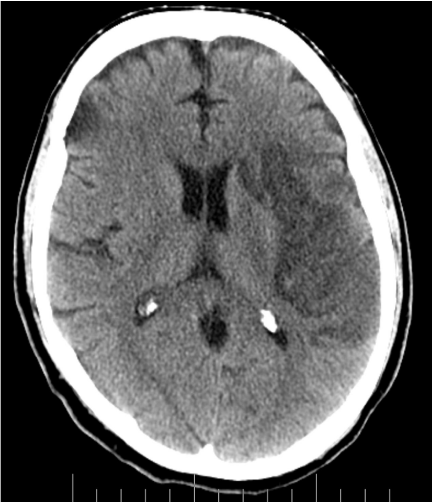
Figure 5 CT- head at 2 weeks- completed left middle cerebral artery infarct with a small stable subarachnoid hemorrhage.
On day 19 an echocardiogram performed showed a less dilated LV and an aortic valve that was opening every other beat. At this point he was transitioned back from Eptifibatide infusion to continuous IV Heparin infusion. His LVAD powers had also decreased during this period compared to admission (Table 1). On day 24 Warfarin therapy was initiated aiming for an INR goal of 2-2.5. Aggressive physical therapy was continued all along. On day 29 he was discharged to inpatient rehab. On day 48 he was discharged home from inpatient rehab on an anti-coagulation regimen that included Aspirin 325mg once dailyand Warfarin (INR goal-2-2.5). On his most recent visit to the LVAD clinic which was day 70 since his initial presentation with the acute stroke, he is able to speak and walk without any assistance or aids. He has mild residual dysphagia but is able to tolerate oral feeds and otherwise has no complaints. His LVAD parameters have returned to baseline (Table 1). He is currently listed and awaiting cardiac transplantation.
Despite the substantial survival benefit associated with LVADs, there is considerable morbidity and mortality associated with LVADs. Device thrombosis and cerebrovascular disease are known feared complications of LVADs.1,2 But in the last decade, with increasing clinical experience with LVADs, better patient selection and improved understanding of anti-coagulation management, device thrombosis and stroke rates have decreased considerably.3 Yet, as seen with the patient in this report and other anecdotal reports (Table 3), device thrombosis still occurs. In a scenario such as with our patient, management is particularly complicated as the patient presented with a clinical picture highly suggestive of a LVAD thrombus complicated by an acute ischemic infarct.
|
Author, Year of publication |
Age, Sex |
Device Make |
Number of days post- LVAD implantation at presentation, postulated explanation for device thrombosis |
Initial LDH IU/L |
Treatment regimen |
|
Tang et al.9 |
51 y/o M |
HM II |
Day#150 Not reported |
Catheter directed- Alteplase 20 mg f/b Alteplase 80 mg IV over 90 minutes |
|
|
Kamouh et al.10 |
62 y/o M |
HW |
Day#18 Sub-therapeutic INR (1.5) |
2400 |
Catheter directed- Alteplase 1mg/min, total 30 mg |
|
Jennings et al.11 |
44 y/o M |
HM II |
Day#121 |
3665 |
Eptifibatide 1mcg/kg/min x 4 days f/bAspirin and Clopidogrel daily |
|
Al-Quthami et al.12 |
66 y/o M |
HM II |
Day#33 Sub-therapeutic INR (1.4) |
2326 |
Heparin x 5 days, f/b Eptifibatide 180µg/kg/min f/b1µg/kg/min |
|
Al-Quthami et al.12 |
25 y/o F |
HM II |
Day#33Sub-therapeutic INR (1.4) |
1292 |
IV Heparin (PTT 60-80) Eptifibatide 180µg/kg/minf/b 2µg/kg/min for 4 days. Warfarin INR (1.5 – 2.5) |
|
Bashir et al.13 |
48 y/o F |
HMII |
Day #3 Not reported |
- |
|
|
Kiernan et al.14 |
57 y/o M |
HW |
Day#150 Sub- therapeutic INR (1.4) |
Catheter directed- Alteplase - 1 mg/min (total dose not reported) |
|
|
Paluszkiewicz et al.15 |
68 y/o M |
HM II |
Day#30 Not reported |
áá |
Lytic therapy (regimen not reported) |
|
Bhamidipati et al.16 |
61 y/o F |
HM II |
Day#18 Excessive early post-operative bleeding requiring multiple blood product transfusions |
>2000 |
Warfarin (INR >2.0) after LVAD exchange |
|
Thomas et al.17 |
38 y/o F |
HW |
Day#140 Not reported |
- |
IV Heparin f/b Tirofiban 0.1 µg/kg/min, ASA 150 mg Clopidogrel 600mg LD, 75mg and Warfarin INR-2.5-3.5 |
|
Meyer et al.18 |
40 y/o M |
HM II |
Day#60 Sub-therapeutic INR |
IV Hirudin PTT 50-60 for 18 days |
|
Table 3 Examples of Thrombus Treatment Regimens with Contemporary continuous flow LVADs Reported in the Literature.M- male; F- female; y/o- years old; HW, Heartware; HM II, HeartMate II; LVAD, Left ventricular assist device, INR, International normalized ratio; PTT, Partial thromboplastin time; LDH, Lactate dehydrogenase; IV, Intra-venous; LD, Loading dose.*Plasma free hemoglobin was checked. Was 0.86 g/liter, an increase from 0.08 g/liter checked 24hours prior. Peaked at 2.7 g/liter and normalized at 0.10 g/liter by Day# .
For our patient, after having a family meeting with the Neurology and LVAD teams and discussing the pros and cons of various treatment options including the risk of hemorrhagic conversion with administration of systemic thrombolysis outside the,4,5 hour window, a decision was made to proceed with systemic thrombolysis. We felt in the setting of an acute stroke, it would be extremely high risk to subject the patient to a device exchange surgery. Regarding Eptifibatide or Heparin infusion, we felt that with significant LVAD power consumption, acute decompensated heart failure, and massive ischemic stroke likely due to embolic source, the patient had an obstructive LVAD thrombus that would require thrombolysis and if left untreated he would be at an extremely high risk for another neurologic event and worsening heart failure.
After administering systemic thrombolysis at presentation, discussions were again held during the course of his hospital course regarding LVAD pump exchange as well as activating him for cardiac transplant but with persistent significant neurologic deficit, we felt he was too critical to undergo a surgery. Also, since he had a good clinical response to systemic thrombolysis with the pump power returning to baseline, resolved acute heart failure decomposition and renal function returning to baseline, we decided to mange him with aggressive anticoagulation and allow him time to recover from the acute stroke before he would be listed for cardiac transplantation or pump exchange. 1 month after his initial presentation he was discharged from the hospital to a rehabilitation facility with his anti-coagulation regimen consisting of Aspirin 325mg daily and Warfarin therapy (goal INR 2-2.5). 2weeks later he was discharged home. Most recently he was seen at clinic, about 2.5months since his acute presentation. He has had no high power alarms on his LVAD or heart failure decomposition episodes. His right hemiplegia has completely resolved with only mild residual dysphagia. He is currently listed and awaiting cardiac transplantation. While our patient had an excellent outcome considering his grave presentation, it again brings up the issue of optimal management of an acute LVAD thrombus. We performed a comprehensive literature search using PubMed, to identify case reports4–18 that relate to thrombus management in patients with continuous flow LVADs. Keywords used for the search included left ventricular assist devices, thrombosis, thrombolytic, Heart Mate II and Heart ware. While we acknowledge that this list (Table 3) is not exhaustive and may not include every published report on acute management of LVAD thrombosis, it certainly illustrates the varying management approaches applied in this situation. Currently it is not clear to care providers if one approach is better than the other (systemic thrombolytic vs. catheter directed thrombolysis vs. LVAD exchange vs. aggressive oral anti-coagulant and anti-platelet therapy) and how mortality and morbidity rates compare between these different approaches. Since LVADs are being more frequently used in treating patients with advanced heart failure, it is crucial for us to gain a better understanding on this serious acute complication. Currently about 150 medical centers participate in the INTERMACS registry that tracks MCS outcomes. Mandating that all LVAD implantation centers (bridge-to-transplant or destination therapy centers) participate in a registry such as this and report complications including patient management in the case of a potential LVAD thrombus would greatly add to our understanding of this very serious complication.
We believe this can be achieved by expanding the coordinated effort between LVAD implanting centers, NHLBI, CMS, ISHLT and LVAD manufacturers.
None.
Authors declare that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.