Journal of
eISSN: 2373-6437


Review Article Volume 14 Issue 6
Assistant Professor, Faculty of Medicine, Department of Anesthesiology and Intensive Care, Indonesia
Correspondence: Aldy Heriwardito, Department of Anaesthesiology and Intensive Care Faculty of Medicine, Universitas Indonesia Jl. Salemba Raya 6, Jakarta, 10340, Indonesia, Tel +6221 4722442, +628 1386211017
Received: November 22, 2022 | Published: December 29, 2022
Citation: Heriwardito A, Aristya L. Tetralogy of Fallot. J Anesth Crit Care Open Access. 2022;14(6):215‒218. DOI: 10.15406/jaccoa.2022.14.00541
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease, represents approximately 7-10% from all congenital heart disease, occurs in 3 to 5 per 10,000 live births.1,2 One epidemiologic study conducted in a referral hospital in Indonesia showed the number of TOF cases in one year reached 7%.3 In 1673, Nicolas Steno founded that TOF was made up of the following four abnormalities of the heart and blood vessels, and its anatomical features further widely studied by Étienne-Louis Fallot in 1888.4 The four defects are as follow.
The narrowing of the valve that connects right ventricle to pulmonary artery results in less blood traveling to the lungs. The narrowest part is located just under the pulmonary valve due to muscle thickening.5
A hole in the septum or wall between right ventricle and left ventricle, most commonly located in perimembranous area and muscles of the septum, causes a shifting of low-oxygenated blood in the right ventricle to the high-oxygenated blood in the left. The defect is commonly large and unrestrictive.2,5
There is a ventriculoarterial connection that builds up above both ventricles, instead of just the left ventricle. The aorta still originates from the left ventricle; however, it is abnormally displaced to the right above the VSD.2,5
A thickening of muscular wall of right ventricle is due to the RVOTO, causing the right ventricle to pump blood out of the heart harder than normally it does.5
The anatomy of TOF causes the blood flowing along the pulmonary and systemic circulation mixed all up together, and the right-to-left shift shunt carries the deoxygenated blood to the systemic circulation. These processes cause cyanosis. The shunt was determined by the relative pressure difference between left and right ventricle, which depends on the degree of RVOTO severity. The pathophysiology depends on the degree of RVOTO. In minimal RVOTO with mild overriding aorta cases, the lesion causes a left-to-right shunt, thus, gives a manifestation of ‘pink tet’. The oxygen saturation may vary, up to 100%. In classic TOF with certain RVOTO degree, a bidirectional flow in VSD shunt commonly happens, and the saturation will go down to 80-90%. Patient can be clinically cyanosis. In severe or complete RVOTO cases, cyanosis is clearly evident and the saturation can be as low as 30%. The severity of right-to-left shunt and the degree of hypoxia will be higher along with the more severe degree of RVOTO and overriding aorta (Figure 1).2
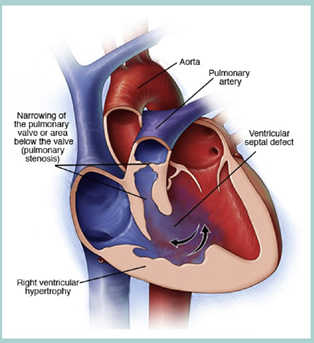
Figure 1 The anatomy of TOF.2
When the patient is agitated and irritated (i.e. crying), an imbalance of circulation occurs and causes sudden desaturation and deterioration. This episode is known as ‘cyanotic spell’ or ‘tet spell’. Tet spell occurs because of the decrease of systemic vascular resistance (SVR) or infundibular muscle spasm, which increases the blood from right to left. The hypoxia will be worse and the carbon dioxide level in blood will be increased, causing the increase of pulmonary vascular resistance (PVR). It results in increase of respiratory effort (tachypnea and hyperpnea) and increase of venous return, thus, worsen the right-to-left shunt. The acute and immediate management of ‘tet spell’ is knee-to-chest position in children to raise the intraabdominal pressure. It will increase SVR and improves the right-to-left shunt.2,6
Diagnosis
Prenatal diagnosis in developed countries is possible using prenatal ultrasonography. Postnatal diagnosis can be done starting from clinical assessment to magnetic resonance imaging (MRI).
‘Hypercyanotic spell’ may occur at any stage of life, depends on the severity of RVOTO. It usually exhibits in infant and can cause syncope or stroke. In older children, they are usually already informed to squat when the episode occurs.
Systolic murmur happens due to a turbulent flow passing RVOT inversely proportional to the degree of obstruction. During ‘hypercyanotic spell’, murmur is often not audible due to the very minimum flow.
Clubbing finger appears in patients with prolonged cyanosis, usually in older children without proper treatment.
Chest plain radiograph will show a boot-shaped heart due to right ventricle hypertrophy and a concave shape in upper left border of heart due to small pulmonary artery.
The electrocardiography will show the sign of right ventricular hypertrophy with right axis deviation.
Echocardiography stays as the gold-standard of diagnosis to assess the heart anatomy.
MRI is considered to be important to evaluate the right ventricle dimension in patients after surgery to determine what next intervention should be done.2
Management
Definitive management of TOF is surgery. Total repair surgery is usually performed to children 6-18 years old. Total repair is a corrective surgical procedure which involves closuring of the VSD with patch, making the aorta only receives blood flow from left ventricle, thinning the right ventricle muscle, and relieving the obstructed pulmonary valve using patch or dilation. If the children do not meet the criteria for total corrective surgery, such as premature infant or pulmonary artery hypoplasia), palliative procedure can be performed. Blalock-Taussig shunt (BT shunt) is a small tube that connects subclavian artery and pulmonary artery to maintain the blood flow to the lungs. Pott shunt is an anastomosis between descending aorta and left pulmonary artery. Waterston shunt is an anastomosis between ascending aorta and pulmonary artery. They are all shunt between aorta and pulmonary artery. The most common performed procedure is modified BT shunt (mBTS) which uses Gore-Tex to connect subclavian artery and pulomary artery. mBTS maintains the blood flow to extremities and prevents inadequate or excessive blood flow to the lungs.2,7
Anesthesia management
Preoperative assessment
Preoperative management of children undergoing palliative or total repair surgery includes history taking, physical examination, the latest transthoracic echocardiography, electrocardiography, echocardiography, and laboratory examination (i.e. complete blood count, coagulation panel, comprehensive metabolic panel). In laboratory examination, polycythemia and mild coagulopathy can be found. Polycythemia with hyperviscosity is the most common underlying pathogenesis causing coagulation. Electrolyte imbalance and low level of blood magnesium can also be found and can be treated with diuretic agents. Echocardiography shows the function and dimension of right ventricle, and also the degree of severity of pulmonary regurgitation.7,9
Anxiolytic
Anxiolytic is an important preoperative management in children. Parents’ presence during induction is not recommended if the children is still approximately 6 months old. However, in older children, premedication before transferring patient to the operating room is given. The most common anxiolytic used is midazolam syrup 0,5 mg/kg orally, given 20 minutes before transfer.7 Other options of anxiolytic premedication are ketamine 10 mg/kg intranasal, morphine 0,2 mg/kg intramuscular, or pentobarbitone 2 mg/kg per rectal.9
‘Tet spell evaluation
Preoperative evaluation of ‘tet spell’, which includes frequency and severity, is very important. Children who previously have not undergone any palliative surgery have higher probability for a ‘tet spell’ to occur during induction. Factors affecting the probability of ‘tet spells’ are dehydration due to prolonged preoperative fasting which decreases right ventricle preload, catecholamine surge in uncooperative children during induction causing RVOT spasm, inhalation agent during induction causing SVR drops, and inadequate ventilation causing hypoxia, hypercarbia, and further PVR elevation.7
Moreover, it is suggested to make interventional cardiologists or cardiovascular surgeons available to perform cardiopulmonary bypass (CPB). CPB performed when ‘tet spell’ occurs during induction will save children’s life if medication therapy does not work. The main objective or ‘tet spell’ management is to release the infundibular spasm and restore the VSD shunt. These maneuvers can be applicated when ‘tet spell’ occurs:7,9
Induction of anesthesia
Before induction, standard monitoring must be readily available. Induction includes induction mask and inhalation agents.7,9 Intravenous ketamine is more recommended previously to maintain SVR and prevent ‘tet spell’. However, the consideration in drug selection and induction method are believed to be less important than careful titration dose and duration of administration, which is performed by experienced anesthesiologist.9 In children who have venous access, intravenous fentanyl and rocuronium with low dose volatile anesthesia agent can be given. Alternative option in children without venous access is intramuscular ketamine and rocuronium.7
Airway management
There is no clear evidence about difficult airway in TOF. However, several TOF cases is a part of chromosomal abnormalities diseases, such as Edward syndrome (trisomy 18), Down syndrome (trisomy 21), and DiGorge syndrome (22q11 deletion), in whom airway difficulties is commonly found. Difficult airway management kit must be readily available in those cases. Tracheal anomaly is found in 11% population and might need smaller endotracheal tube.9
In mBTS procedure, perioperative and postoperative mechanical ventilation requires endotracheal tube. In children under 18 months, nasotracheal tube can be considered to reduce the possibility of tube detachment, especially during intraoperative transesophageal echocardiography (TEE) and transfer to intensive care unit (ICU).
Transfusion and anticoagulant management, position, transfer, and mobilization
In cyanotic patient, polycythemia and mild coagulopathy can be seen. Patients with previously underwent BT shunt can be given aspirin. Patients with mechanical valve replacement surgery can be given anticoagulant. There is no specific position, transfer, and mobilization technique required perioperatively. In patients experiencing ‘hypercyanotic spell’, knee-to-chest position is required to increase SVR, decrease shunting, and improve cyanosis.9
Vascular access
Central arterial and venous access is recommended in all patients. Arterial access is preferably through femoral artery because subclavian artery will be clamped during surgery. Central venous access is used to administer inotropic and fluid management.7,9
Antithrombotic agent
Low dose heparin, 50-100 unit/kg, sometimes is given during shunting to minimize the risk of thrombosis.
Fluid and vasopressor
When the shunt is opened, blood flow to pulmonary artery during diastolic causing drop in blood pressure, so fluid and vasopressor are needed. In two lungs ventilation, systemic oxygen saturation of 80% is considered optimal, which indicates a balance between systemic and pulmonary blood flow. Lower oxygen saturation signifies any ventilation or shunt problem, or low cardiac output.7 In patients undergoing total repair, anesthesia technique must follow standard technique implemented during CPB surgery. TEE must always be used, before and after CPB, to evaluate the success outcome of surgery and to evaluate the cardiac function.7
Postoperative management
Postoperative management after mBTS must be done in ICU. Low dose heparin starting from 10 U/kg/h immediately after surgery is given to maintain the shunt patency. If patient is ready with enteral feeding, it can be continued with giving low dose aspirin to prevent shunt occlusion until the definitive total repair surgery can be performed.7
In patient undergoing CPB procedure, dopamine and milrinone are needed to improve right ventricle dysfunction. Milrinone, given as a loading dose of 50-75 mg/kg in 30 minutes followed by 0,5-0,75 mg/kg/min infusion, can reduce the risk of low-cardiac-output syndrome in children who previously have undergone other congenital heart surgery. Arrhythmia and block commonly occur in VSD surgery because the patch is placed near conduction system. Epicardial pacing might be needed, however, it is temporarily used until the edema surrounding the VSD patch dies down. Epicardial pacing needed for more than 10 days may indicate the need for permanent pacing.
Junctional ectopic tachycardia (JET) can occur after TOF surgery. Milrinone and dopamine are possible risk factors, with the signs of heart ratee 180-260 beat per second, narrow QRS complex in electrocardiogram, and atrioventricular conduction dissociation. This accelerated heart rate is difficult to be tolerated if the systolic and diastolic function are poor. Magnesium, surface cooling, and amiodaron can help to improve patient’s condition.7 Continuous monitoring in ICU must be done with the help from cardiologist which is expert in congenital heart disease.9
Complication
Complication of operative surgery of TOF as follow.
TOF is one of the most common congenital heart diseases. Definitive treatment includes palliative and total repair surgery. Anesthesia consideration is based on the goal of perioperative management, preventing the increase of right-to-left shunt which causing ‘hypercyanotic spell’. Therefore, all anesthesia management are strived not to decrease SVR or increase PVR.
None.
None.

©2022 Heriwardito, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.