Journal of
eISSN: 2373-6437


Research Article Volume 15 Issue 3
1Hospital General de Zona del Instituto Mexicano del Seguro Social No. 252, México
2Consultorio dental privado San Andrés Tuxtla, México
3Hospital San Ángel Inn Chapultepec ciudad de México, México
4Hospital General de Zona del Instituto Mexicano del Seguro Social No. 33, México
5Centro Médico ISSEMyM Toluca, México
6Hospital Ángeles del Carmen, México
Correspondence: Vilchis-Valentin David, camino flor de María y calzada sr del huerto #154 colonia san Martín Atlacomulco de Fabela C.P. 50640 Estado de México , Tel 5554165470
Received: June 22, 2023 | Published: June 30, 2023
Citation: Vilchis-Valentin D, García-Maldonado M, Larrazolo-Ochoa A, et al. Systematized review of the literature on postoperative nausea and vomiting. J Anesth Crit Care Open Access. 2023;15(3):101-107. DOI: 10.15406/jaccoa.2023.15.00561
Introduction: Postoperative nausea and vomiting (PONV) is the most common adverse effect, after postoperative pain, with an incidence of 31.1% to 80%, which increases pain, favors broncho aspiration, wound dehiscence, and hematoma formation.
Methodology: Systematized search keywords, postoperative nausea and vomiting, Incidence, therapeutics, Apfel score and risk score for postoperative nausea and vomiting, in PubMed database, the Cochrane central register of controlled trials and in http://www.clinicaltrials.gov.
Results: A total of 2750 articles were obtained, and 62 articles were chosen for inclusion.
Discussion: The drugs used as monotherapy to mitigate PONV such as palonosetron, fosaprepitant and aprepitant, show better results than the rest of the drugs.
Conclusion: Currently, NK1 receptor antagonist drugs and 5-HT3 antagonists have been shown to have the best results in preventing PONV, however, the management of PONV should be multimodal and individualized.
Keywords: postoperative nausea and vomiting, incidence, therapeutics, Apfel score and risk score for postoperative nausea and vomiting.
Postoperative nausea and vomiting (PONV) are the most common adverse effect after postoperative pain, with an incidence ranging from 27.1 to 80%.1,2 PONV has a negative impact on the patient's recovery, increasing pain, favoring bronchial aspiration, wound dehiscence, hematoma formation, increasing the patient's distress and increasing the number of days of hospitalization, generating unforeseen expenses to mitigate or control the symptoms.3 In surveys carried out in the United States of America, patients have stated that they are willing to spend up to 100 dollars to prevent PONV.4
PONV has a complex pathophysiology, and the prevalence of this adverse effect has not been reduced, despite multiple interventions to prevent its occurrence.4 The recommendations to prevent PONV still have many discrepancies, which is why studies on the risk of PONV and more effective pharmacological interventions continue to be carried out.
A systematized search with keywords, postoperative nausea and vomiting, Incidence, therapeutics, Apfel score and risk score for postoperative nausea and vomiting, was performed in the pubmed database, the Cochrane central register of controlled trials (CENTRAL) and in http://www.clinicaltrials.gov for clinical trials that are still in progress.
Randomized clinical trials, meta-analyses, systematized reviews, prospective observational and retrospective studies, as well as incidence studies were included for reading and analysis, the search was limited to humans older than 19 years and publications from 2017 to 2022. Two independent pubmed searches were performed with the phrase postoperative nausea and vomiting, pathophysiology from 2015 to 2022 and another with the phrase evaluation of risk scores to predict postoperative nausea and vomiting from 2000 to 2022.
From the initial search in pubmed 266 articles were obtained and from the Cochrane database 2484 articles were obtained; a total of 2750 articles were obtained when both databases were combined. Of these, a review by titles was carried out and 2106 were eliminated since they did not include postoperative nausea and vomiting, they were studies in animals such as rabbits or mice, repeated studies or studies that were still in progress without preliminary results, leaving a total of 644 articles for reading. When a new screening was performed, 586 articles were eliminated because they were studies in a population under 19 years of age or were focused on palliative care, in addition to an outdated Cochrane article that the authors abandoned and the publication was withdrawn, leaving a total of 58 articles for this systematized review, additionally, 4 articles are added, 3 of these are the result of the independent search for the pathophysiology of PONV and one that compares 3 scales for the risk of PONV; obtaining a total of 62 articles for this review. Of the 62 articles, 12 are meta-analyses, 5 are systematized reviews, 25 are clinical trials, 3 are on prevalence and incidence, 12 are prospective observational studies, 2 are retrospective observational aggregate studies, and 3 descriptive articles on PONV pathophysiology are included (Diagram 1).
Incidence
Experiencing PONV has a negative impact on the patient's experience, reducing the level of satisfaction with medical care and significantly increasing the costs of mitigating symptomatology, prolonging hospital stay and hindering patient recovery.5,6 The incidence of PONV is variable and depends on each hospital center; there are reports that this incidence ranges from 27.1 to 80% of post-surgical patients.1,2 With the discrepancy in the incidence, it has been necessary to conduct in-depth studies regarding factors other than those included in the Apfel scale that increase or decrease the risk of PONV; examples of factors that increase the risk of PONV are laparoscopic, head and neck surgeries, as well as gynecological surgeries, in addition to the duration of anesthesia and/or surgery of more than 3 hours.1,2,5
Pathophysiology
Thomas Wiesmann et al. have described the neuronal circuits involved in emesis, mentioning that there are many peripheral and central stimuli directed to the rhombencephalon caudal, capable of triggering an emetic episode. Colloquially we speak of a vomiting center, however, it is not an isolated anatomical structure, but an area made up of the nucleus of the solitary tract (NTS) and nuclei of the reticular formation, which may be key players in the "functional vomiting center". Signals from the area postrema (AP) and the vestibular nerve may activate emesis through direct projections to the NTS. The AP is an organ with a reduced blood-brain barrier, which serves as a detector of circulating toxins. According with the previous idea, it is concluded that the stimuli that provoke nausea and vomiting originate from the visceral afferent, vestibular and chemoreceptor areas (Figure 1).4,7
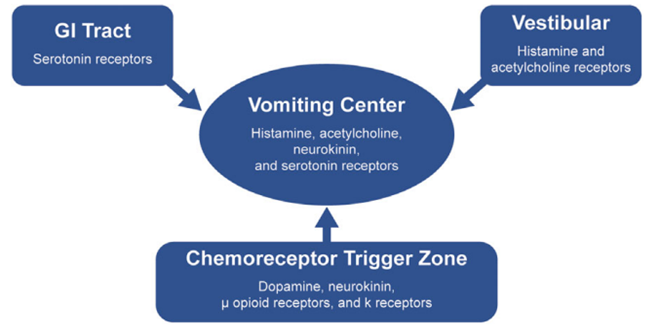
Figure 1 Pathophysiology of opioid-induced nausea and vomiting. Theresa Mallick, SearleMechele Filmen 2017.
The pathophysiology of nausea is less well known, and it is proposed that areas such as the parabrachial nucleus of the dorsal pons, thalamus and amygdala are areas involved in the genesis of nausea, with an additional role of the sympathetic nervous system (SNA) that causes the physiological changes of sweating, pallor, salivation, increased blood pressure, tachycardia, cutaneous vasoconstriction, and decreased gastrointestinal motility.7 It has been proposed that everyone has a threshold for nausea, which changes from minute to minute; this change is due to psychological factors of the individual, such as anxiety and adaptive capacity.4,7
Dopamine 2 (D2) receptor stimulation produces activation of adenylate cyclase, which in turn alters the amount of cyclic adenosine monophosphate (cAMP) within neurons in the NTS and AP, causing nausea and vomiting, in addition to this, 5-hydroxytryptamine (5-HT3) receptor activation in the AP and intestinal vagal afferents also causes vomiting, while histamine type 1 (H1) receptor blockade has been shown to reduce PONV symptoms; however, the signaling pathway that generates nausea or vomiting mediated by this receptor is still unknown; with respect to corticosteroid receptors, it has been found that they have an anti-inflammatory effect in the NTS, causing nervous desensitization, this being the possible explanation for their clinical efficacy in PONV; finally, it has been described that blocking the neurokinin 1 (NK1) receptor, in the NTS and in the reticular formation, mitigates the symptomatology of PONV; this receptor is a highly relevant therapeutic target, since drugs that act at this level may have greater efficacy than other drugs in preventing PONV (Figure 2).4

Figure 2 pathophysiology of postoperative nausea and vomiting. Thomas Wiesmann, Peter Kranke & Leopold Eberhart 2015.
Opioids induce emesis by direct action on mu (µ) receptors in the AP, and by direct stimulation of the vestibulocochlear apparatus, through the activation of µ, kapa (k), delta (d) receptors and altering gastrointestinal motility; in addition, opioid binding to µ receptors causes intestinal distension and vomiting.6 Inhaled anesthetics such as nitrous oxide, sevoflurane and desflurane interact with acetylcholine, gamma-aminobutyric acid (GABA), N-methyl-D-aspartate (NMDA) and opioid receptors in the central nervous system (CNS), as well as at the peripheral level, causing alterations in gastric motility, triggering nausea and vomiting.4,7,8
Presenting PONV is an unpleasant experience, involving cognitive and emotional centers; This has been demonstrated by means of magnetic resonance imaging where stimulation of the medial prefrontal cortex and anterior cingulate cortex causes an episode of nausea and increased heart rate,6 in addition, it has been found that kinetosis shares the same signaling pathway as fear and emotional agitation at the level of the amygdala, putamen and locus coeruleus, suggesting that this is the reason, which causes stress and fear of reoccurrence of PONV. Finally, the anterior cingulate cortex, insular cortex, nucleus accumbens, amygdala and medial prefrontal cortex, which are areas involved in acute and chronic pain, are also part of the nausea circuit, suggesting that pain may be a cause of nausea and vomiting.7,8 Considering all the structures involved, we can find a relationship between PONV, memory and conditioning created by the patient before these adverse effects, which means that even before presenting an episode of PONV, the mere thought of it magnifies the sensations, generating stress and anxiety.
Risk factors and predictive scales for PONV
Creating a scale that predicts PONV continues to be a challenge; for this reason, there is more than one scale that tries to calculate this risk in a simplified and useful way; however, there is no scale that is 100% accurate, since the genesis of PONV will depend on factors inherent to the patient and factors inherent to the surgical procedure.9 Koivuranta and Apfel identified some factors that gave rise to the staging systems for the risk of PONV, including being female, having a history of PONV/dizziness or motion sickness, non-smoker, the use of postoperative opioids and the duration of the surgery or anesthesia (Table 1).10 Despite considering the factors mentioned above, other factors have been found to contribute to the occurrence of PONV, such as being a young patient, use of volatile anesthetics, head and neck surgery, laparoscopic surgery and gynecological surgery.11
|
Risk Factors |
Percentage of risk received according to Apfel scale. |
|
No risk factors. |
10% |
|
Being woman |
20% |
|
Non- smoker |
20% |
|
History of kinetosis or PONV. |
20% |
|
Postoperative opioid use |
20% |
Table 1 Risk factors included in the simplified Apfel scale to determine the risk of presenting PONV for each patient.
Considering the great problem of PONV, several scales have been developed to try to predict its occurrence; for this reason, in 2000 Eberhart et al. compared three available scales to determine the risk of suffering PONV: Palazzos, Koivuranta and Apfel, the results support the hypothesis that individual risk factors have an impact on the occurrence of PONV, with the risk score presented by Koivuranta and Apfel performing better than the Palazzo score, with the Koivuranta scale being the most favored.12 Currently, the simplified Apfel scale is one of the most widely used in clinical practice, due to the simplicity with which it can be applied in the anesthetic assessment consultation; this scale stipulates that a patient who has no risk factors is given a 10% probability of presenting PONV, while those who present one, two, three or four risk factors are given a probability of presenting PONV of 20, 40, 60 and 80% respectively (Table 2).10
|
Risk factors |
Estimated risk percentage for PONV. |
Number of drugs used |
|
No risk factors. |
10% |
5-HT3 antagonist or dexamethasone. |
|
1 o 2 risk factors |
20%-40% |
Administer 2 pharmacologic agents. |
|
3 o 4 risk factors |
60%-80% |
. Administer 3 or 4 pharmacologic agents. |
Table 2 Staging of risk factors and number of drugs recommended to be administered for each number of accumulated risk factors.
In order to stage PONV and thus make prevention more appropriate, it is advisable to apply a scale such as the Apfel, which despite having a sensitivity and specificity of 65 and 70%,13 should be completed with other independent risk factors, such as the type of surgery to be performed, if it is laparoscopic surgery, since patients undergoing these procedures, despite medication with ondansetron, up to 24% of them present PONV and when ondansetron plus dexamethasone are combined, an improvement is seen, however, up to 8% present PONV14 and in the context of outpatient laparoscopic surgery it is reported that 1 out of 4 patients present PONV.15 When analyzing laparoscopic interventions, such as gastrectomy, the important role played by the stomach in the pathophysiology of PONV has been elucidated, since patients undergoing laparoscopic gastrectomy present more PONV than patients undergoing other types of laparoscopic interventions, making it evident that the stomach is an organ with autocrine, paracrine, and endocrine functions, which help to reduce the appearance of PONV. It should be emphasized that patients who have a nasogastric tube in place are at greater risk of presenting PONV than those who do not.16 On the other hand, in neurosurgery, an incidence of 22 to 70% PONV without prophylaxis has been estimated,17 while with other head and neck surgeries, mainly ear surgery, the presence of PONV has been estimated at 60 to 80% without prophylaxis,18 while orthognathic surgery reports a higher incidence of PONV compared to minor surgeries of the oral cavity, such as dental extractions.19
In hysterectomy, an incidence of 42.9% has been estimated, despite enthymematic prophylaxis with palonosetron and 52.9%, despite receiving ondansetron,20 concluding that gynecological and lower abdominal surgery have 6.27 and 4.56 times the risk of PONV.10 In cardiac surgery, the prevalence of PONV has not been estimated, but it has been shown that 4mg of betamethasone and 0. 625mg of triperidol have had good results in preventing PONV, without significant side effects, in fast track anesthesia protocols, antiemetic medication before and after cardiac surgery is not necessary, since the drugs used during the trans-surgical period have good results; on the other hand, with robotic mitral valve replacement surgery, intense pain and PONV are common in up to 67% and 77% respectively.21-23 The use of halogenated agents during general anesthesia increases the risk of presenting PONV by 30%,24 other points to consider are the duration of the anesthetic surgical procedure (greater than 3hrs), perioperative fasting (greater than 8 hrs.), BMI >30kg/m2, migraine, gastroesophageal reflux or regurgitation, also to psychological factors, such as anxiety.25,26 Eckhard Mauermann et al. found that a higher dose of intraoperative fentanyl was associated with PONV, despite the use of total intravenous anesthesia (TIVA) with propofol; because of this, they recommend the inclusion of intraoperative fentanyl in the Apfel scoring scale.27 Although there is little evidence on the role of cannabis use in PONV, Suhre Wendy et al. report that the risk of PONV is 3.3% (95 % CI: 0.4-6.4 %).28
Pharmacological management
With the current evidence, hospital centers have established guidelines for risk staging and management of PONV, however, these strategies are limited by inadequate involvement of anesthesia providers, who are responsible for completing assessments and administering PONV prophylaxis.29,30 Once the risk for PONV has been established, it is recommended that patients with 1 or 2 risk factors receive 2 pharmacological agents as a preventive measure; when the patient has more than 2 risk factors, 3 to 4 drugs with different sites of action should be given to prevent PONV;13 the selection of drugs is at the discretion of the anesthesiologist. It should be noted that anesthesiologists have had an 89.6% adherence to these recommendations, so the importance of implementing these strategies needs to be made known.31
Of the drugs used to prevent PONV, 5-hydroxytryptamine-3 (5-HT3) antagonists stand out, including ondansetron, palonosetron, ramosetron, granisetron, dolasetron, among others; each of them has an efficacy in preventing PONV, and ondansetron was found to have an RR of 0.55 for prevention of PONV, while ramosetron has an RR of 0.44 and granisetron of 0.45.6,11,32 In adult women undergoing gynecological laparoscopic surgery, palonosetron and granisetron are superior to ondansetron in the prevention of PONV;3 in contrast, palonosetron is more effective after general anesthesia in non-gynecological laparoscopic surgery than granisetron, due to the fact that palonosetron has a longer half-life;33 furthermore, the side effects of these drugs have been statistically insignificant.34,35
Jung et al. mention that multimodal pain management in total knee arthroplasty often causes postoperative vomiting and that patients treated with palonosetron have a lower incidence of PONV during the first 48 h.36 On the other hand, in patients undergoing live related renal transplantation, palonosetron and ondansetron have shown an adequate safety profile for the prevention of PONV, with no overall serious adverse effects.37 Haloperidol and droperidol. are dopamine antagonist drugs used for the management of PONV, with respect to haloperidol it has been found that it can reduce intraoperative nausea RR 0.38; likewise, it can reduce intraoperative vomiting episodes RR 0.41, while postoperative nausea decreases RR 0.61 and for postoperative vomiting, they are recommended since they reduce emesis events RR 0.63,32 with respect to droperidol it has been found that it presents a preventive effect on PONV RR of 0. 61.6
Atypical antipsychotics are currently a group of drugs under study for the management of PONV, since they have an effect on dopamine, alpha-adrenergic, serotonergic, cholinergic and histaminergic receptors,38 which are involved in the pathophysiology of PONV; chronic administration of olanzapine, aripiprazole and risperidone has been shown to decrease the need for an antiemetic in the post anesthesia care unit; despite these findings, the efficacy and safety profile in the prevention of PONV remains to be determined.39 In clinical trials conducted by Peter Kranke et al, amisulpride 5mg+ondansetron or intravenous dexamethasone, compared to placebo, the incidence of emesis was 13.8% in the amisulpride plus ondansetron or dexamethasone group, compared to 20% in the placebo group (P=0.003), for nausea, the incidence was 50% in the intervention group, as opposed to 58.3% in the placebo group (P=0.002) and rescue medication was used in 40.9% of the intervention group, compared to 49.4% of the placebo group (P=0.002), concluding that amisulpride has a good protective effect for PONV;40 similarly, Jaime Hyman et al. report that when ondansetron and dexamethasone are combined with olanzapine, the risk of nausea and/or vomiting decreases by 60% in the first 24 hours after outpatient surgery.41
The use of corticosteroids to prevent PONV has been very controversial, however, they are widely used, within this group of drugs, dexamethasone has shown to reduce intraoperative nausea and vomiting RR 0.56 and 0.52 respectively, with respect to postoperative nausea and vomiting, it shows a decrease in its incidence RR 0.59 and 0.68.32 In studies performed in total hip replacement surgeries, intravenous glucocorticoids decrease pain intensity and PONV after surgery.42,43
Grape et al. tested the efficacy of dexamethasone for PONV in patients who received neuraxial opioids, concluding that its administration mitigates the need for rescue medication RR 0.44;44 in addition, adding a second drug to prevent PONV has shown greater efficacy than dexamethasone monotherapy, which is evident in patients undergoing laparoscopic gastric sleeve surgery, this is evident in patients undergoing laparoscopic gastric sleeve surgery, where mirtazapine+dexamethasone and aprepitant+dexamethasone have had better results in reducing PONV than corticosteroids alone.45 In the study by Ghodrat et al, where they used fentanyl 1mcg/kg+ dexamethasone 16mg, they found a significant decrease in pain and PONV, compared to those who received fentanyl alone.46
Dexamethasone is used to reduce the risk of PONV, and the results obtained from its use are greater than placebo; however, these results are better when combined with another antiemetic drug; It is important to highlight that corticosteroids are ineffective in aborting retching or nausea.45,47 The most used antihistamines for the prevention of PONV are dimenhydrinate and pyridoxin, which have little or no effect on intraoperative nausea RR 0.99, while postoperative nausea is effectively reduced RR 0.44; however, it is not known whether antihistamines reduce postoperative vomiting RR 0.48.32 The anticholinergic scopolamine is used to prevent PONV, however, it has a patch presentation and should be placed one night before surgery. This drug reduces intraoperative nausea RR 0.67, with little or no effect on intraoperative vomiting RR 0.79, however, postoperative vomiting is reduced RR 0.55.32 Another drug used is glycopyrrolate, which has shown superiority in preventing PONV over dexamethasone alone; however, on its own, it does not surpass the efficacy of ondansetron, so its use is suggested as an adjuvant and not as monotherapy.48
The sedative drugs used for the management and prevention of PONV are midazolam, dexmedetomidine and propofol, which reduce intraoperative nausea RR 0.65 and intraoperative vomiting RR 0.35; however, it is not yet clear whether they reduce postoperative nausea RR 0.25, but they can reduce postoperative vomiting RR 0.09.32 Midazolam has similar efficacy to the combination of ondansetron dexamethasone; however, it is suggested to use midazolam with other antiemetics, due to its short elimination half-life.49 Recently, the effects of dexmedetomidine on PONV in patients undergoing laparoscopic surgery have been investigated, concluding that the use of dexmedetomidine in postoperative infusion has antiemetic effects in the early postoperative period (less than 6 hours).50 With respect to the use of propofol infused at 1.0 mg/kg/h, after a loading dose of 0.3 mg/kg, it is concluded that it reduces the incidence of PONV; these results are more efficient when another antiemetic is administered, such as dexamethasone or a 5-TH3 antagonist, since this minimizes the use of rescue antiemetics.51,52 In the meta-analysis by Anwar et al, they showed that gabapentin, administered as a single preoperative dose, significantly reduces postoperative nausea and vomiting in shoulder arthroscopy.53
NK1 receptor antagonists such as aprepitant and fosaprepitant, in clinical trials have similar efficacy to most of the combinations performed to prevent PONV RR 0.26.6,11 In morbidly obese patients undergoing laparoscopic gastrectomy, the combination of aprepitant/dexamethasone is superior to dexamethasone alone in relieving postoperative nausea and vomiting;45 the great disadvantage of this group of drugs is their high cost.
Other strategies with the potential to prevent PONV have been investigated, among them paracetamol, a drug used mainly as an analgesic and antipyretic, but recently it was found to have a positive effect in the prevention of PONV, as demonstrated by Moham et al., who reported that the use of paracetamol alone has the same effect as the mixture of paracetamol plus dexamethasone, demonstrating its preventive effect on PONV.54 Another innovative technique that continues to be studied is the intrathecal administration of atropine sulfate + hyperbaric bupivacaine + morphine; the results obtained with this mixture have concluded that there is an antiemetic effect that does not affect postoperative analgesia;55 however, studies are lacking to determine the safety of this combination, so its use should be carried out with great caution. With respect to pharmacological lysis of the neuromuscular blocker, Nisn et al. concluded that the use of sugammadex has a lower incidence of PONV than that observed with neostigmine + atropine.56
In addition to pharmacological therapy, recent studies are assessing the impact of fluid therapy in the prevention of PONV, finding that the use of crystalloids in ASA I and II patients receiving general anesthesia reduces the risk of postoperative nausea,57 RR 0.62 (p < 0.00001) and a preventive effect of vomiting during the first 24hrs post-surgery.58 Another fluid therapy strategy for PONV is the administration of colloids, which has had a superior effect in preventing PONV than the administration of crystalloids in patients undergoing lower abdominal surgery who received general anesthesia for 3 hours or more; however, these results are not the same in patients who received general anesthesia for 3 hours or more, however, these results are not the same in patients who received general anesthesia for less than 3 hours.59 On the other hand, the administration of bolus glucose solution is still very controversial, since some results show that it does not reduce PONV,60 while Chisaki et al. report its usefulness in reducing postoperative nausea, but not vomiting.61
These last fluid therapy recommendations should be handled with caution, since the risk of serious adverse events resulting from the supplementary administration of intravenous crystalloids and colloids is unknown, and it is not clear whether patients are at risk of being readmitted to the hospital postoperatively due to these interventional measures.57,58 Finally, multimodal management of PONV is recommended, even from the preoperative period, since oral premedication with non-opioid drugs, such as paracetamol and gabapentin, has better results than medication of patients during surgery; multimodal management of PONV aims to improve the care and experience of the patient who is going to undergo surgery.62
PONV continues to be a very frequent adverse event that impacts patient recovery and generates an aberration towards surgical/anesthetic procedures; the genesis of this adverse effect has multiple triggers, which can be inherent to the individual, such as gender, age, being a smoker or not, as well as the surgical-anesthetic procedure performed, such as general anesthesia for more than 3 hours, use of high doses of opioids during and after surgery, head and neck surgery, laparoscopic or gynecological surgeries. It should be considered that this adverse event not only impacts on the patient, but also generates unwanted hospitalization expenses.
The drugs used as monotherapy to mitigate PONV, such as palonosetron, fosaprepitant and aprepitant, have the best results compared to the rest of the drugs, although access to them is difficult, for this reason, it is recommended to continue using the drugs to which access is available, It is suggested to choose them according to the existing guidelines, in which, if an individual does not present risk factors of the Apfel scale, he/she will have a 10% chance of suffering PONV, to them it is suggested the administration of a 5-HT3 antagonist drug or dexamethasone; if the patient has 1 or 2 risk factors, they will have up to a 40% chance of manifesting PONV and the administration of 2 pharmacological agents is suggested; finally, if the individual has more than 3 risk factors, it is pertinent to give them 3 or 4 pharmacological agents, because these patients have a 60 and 80% chance of having PONV; Table 2. Current guidelines for the prevention of PONV are aimed at multimodal management, performing pharmacological interventions such as the administration of preoperative gabapentin, to the supplementary use of crystalloid boluses and intravenous colloids; in both cases there is still a lack of evidence and the complications that patients may develop from these interventions have not been determined, therefore, their use cannot be routinely recommended. Although dexamethasone is useful to prevent PONV, it is the least effective drug, compared to the rest of the drugs used for the prevention of PONV, considering the above, it is suggested that it be administered together with other drugs for the prevention of PONV; in addition to the above, the use of diphenidol is also advised in those patients who have motion sickness as a risk factor for PONV.
The fact that PONV has a complex pathophysiology with diverse causes and risk factors, making its prevention and management difficult, when considering all the pathways involved in the genesis of PONV, makes it evident why there is no single drug that can eliminate this adverse effect, however, However, it has currently been demonstrated that NK1 receptor antagonist drugs and 5-HT3 antagonists have the best results in preventing PONV, however, by themselves they are not 100% effective, so it is suggested to estimate the risk of presenting PONV on an individual basis, in order to determine the number of antiemetic drugs that should be received. It should be emphasized that the management of PONV should be multimodal and individualized, since there are factors independent of those established by Apfel that favor the appearance of PONV.

©2023 Vilchis-Valentin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.