Journal of
eISSN: 2373-6437


Case Report Volume 16 Issue 3
1Unit of Pediatric Intensive Medicine, Mother and Child University Hospital of the Canary Islands, Spain
2Faculty of Health Sciences, University Fernando PessoaCanarias, Spain
Correspondence: López-Álvarez JM, Unit of Pediatric Intensive Medicine, Mother and Child University Hospital of the Canary Islands, Las Palmas de Gran Canaria, Spain
Received: May 12, 2024 | Published: July 15, 2024
Citation: Caballero-Estupiñán E, López-Álvarez JM, Lorenzo-Villegas DL. Severe methemoglobinemia in a 10-month-old patient in the pediatric intensive care unit. case report. J Anesth Crit Care Open Access. 2024;16(4):88‒90. DOI: 10.15406/jaccoa.2024.16.00596
Methemoglobinemia is a condition characterized by elevated levels of methemoglobin (>1.5 g/dl) in the blood, leading to tissue hypoxia and cyanosis. This disorder can range from asymptomatic to severe, with manifestations such as altered mental status and generalized seizures. In children, increased oxidation of hemoglobin's heme group is ften due to exposure to nitrates found in certain foods and drugs, compounded by infants' biochemical peculiarities. Prompt recognition and appropriate management are essential to prevent complications. Management involves identifying and eliminating triggers, supportive care, and specific treatments such as methylene blue. However, caution is warranted in patients with glucose-6-phosphate dehydrogenase deficiency, as methylene blue can precipitate hemolysis. Ascorbic acid may be considered as an alternative in such cases. We present a case of a 9-month-old infant admitted to the intensive care unit with severe methemoglobinemia, likely triggered by dietary nitrate exposure. Treatment with methylene blue effectively reduced methemoglobin levels, resolving cyanosis and metabolic acidosis.
Keywords: Methemoglobinemia, cyanosis, methylene blue, pediatrics
Methemoglobinemia is defined as an increased presence of methemoglobin in the blood (>1.5 g/dl) produced by oxidation of the iron in the heme group of hemoglobin from ferrous ion (Fe2+) to ferric ion (Fe3+) causing tissue hypoxia and cyanosis.1 The form of presentation can range from asymptomatic to cyanosis (the most common clinical form) and in severe cases can cause altered mental status, coma and generalized seizures.1 In children, the cause of the increased oxidation of the heme group is usually due to the oxidizing capacity of nitrates.1-3 These are present in various foods, especially vegetables (chard, spinach, lettuce) as well as in other substances, including drugs as nitric oxide. Added to this, there are the biochemical particularities of infants younger than one year. Therefore, foods such as chard and spinach should not be introduced into the infant's diet until after the age of one year, due to their potential oxidizing power. Likewise, poor or prolonged preservation of cooked vegetables constitutes a methemoglobinizing risk factor.4-6
When acute methemoglobinemia is suspected, the treatment of choice if the patient is asymptomatic and the methemoglobin concentration is less than 30% is the administration of oxygen. If symptoms appear or the methemoglobin exceeds 30%, intravenous methylene blue 1-2 mg/kg should be administered except in specific cases which will be explained later.6,7
Presentation of the case
A 9-month-old boy, with no medical history of interest, admitted to the intensive care unit due to acrocyanosis and perioral cyanosis, blood oxygen saturation < 90% despite oxygen therapy and a blood gas methemoglobinemia value of 35% (severe methemoglobinemia). No other symptoms, previous infectious conditions or family epidemic environment. No intake of drugs or toxic products, or green leafy vegetables, but they do report eating pumpkin, sweet potato, courgette and broccoli several times a week.
After confirming the diagnosis of methemoglobinemia, additional tests (blood count, biochemistry, coagulation, and blood gas analysis) were performed. All were normal but blood gas analysis because of methemoglobinemia value. Methylene blue was administered at 1.4 mg/kg and the patient was transferred to intensive care, where he remained hemodynamically stable, reactive, with normal physical examination. Progressively, cyanosis resolved and blood oxygen saturation increased, so oxygen therapy was gradually reduced and serial blood gases were taken, showing a progressive decrease in methemoglobinemia to 1.5% and resolution of metabolic acidosis and hyperlactacidemia with only one dose of methylene blue, so oral tolerance was started and the patient was transferred to the ward for follow-up and clinical control.
Methemoglobinemia is an alteration of oxygen transport in the blood that causes tissue hypoxia. Values greater than 1.5 g/dl are considered pathological. Pathophysiology is characterized by an increased formation of methemoglobinemia in the blood, due to oxidation of the heme iron from its ferrous form (Fe2+) to the ferric state (Fe3+). This decreases the affinity of hemoglobin for oxygen and shifts the hemoglobin dissociation curve to the left, thus reducing oxygen release to tissues and causing tissue hypoxia.1,8
Under normal conditions there is minimal formation of methemoglobinemia (1% of total hemoglobin) due to hemoglobin oxidation-reduction equilibrium. Oxidation occurs when hemoglobin reacts with endogenous free radicals or external oxidizing chemicals, leading to increased formation of methemoglobin. This methemoglobin is reduced by the enzyme cytochrome b5 reductase (Cyb5R) which is responsible for reducing the ferric ion in methemoglobin to ferrous ion in hemoglobin by electron transfer by the enzyme NADH. The pathology occurs when there is an imbalance between these two forces.8
The causes can be both genetic and acquired. Genetic causes include deficits in cytochrome b5 reductase enzyme activity, with a distinction between types I and II, as well as variants of hemoglobin M. Acquired causes, on the other hand, include exposure to various drugs, chemicals and foods, with an emphasis on nitrates and nitrites present in foods such as green leafy vegetables and certain drugs such as topical anesthetics and antimalarials.9-11
Acquired causes are listed in Table 1.1,4,11
|
Drugs |
|
|
Amino salicylic acid |
Clofazimine |
|
Chloroquine. Primaquine |
Dapsone |
|
Local anaesthetics: lidocaine, benzocaine, prilocaine |
Menadione |
|
Methylene blue |
Metoclopramide |
|
Nitric oxide (between 5 and 80 is ppm rare) |
Rasburicase |
|
Nitroglycerin |
Phenacetin |
|
Phenazopyridine |
Sulphonamides |
|
Quinones |
|
|
Food |
|
|
Well water Green leafy vegetables (chard, spinach, lettuce, broccoli) |
Frozen or dehydrated food |
|
Mushrooms |
Root vegetables (carrot, ginger, onion) |
|
Green leafy vegetables (chard, spinach, lettuce, broccoli) |
|
|
Chemicals |
|
|
Acetanilide (varnishes, gums, dyes) Resorcinol (wood, resin) |
Chlorates and chromates (Industrial chemistry) |
|
Antifreeze |
Benzene-derived solvents |
|
Hydrogen peroxide |
Naphthalene and naphthoquinone |
|
Sodium nitrate. Other nitrates. |
Nitrogen fertilizers |
|
Solvents (nitrobenzene) |
Herbicides (paraquat) |
|
Resorcinol (wood, resin) |
|
Table 1
It should be noted that infants are more susceptible to methemoglobinemia in the face of these external factors due to decreased methemoglobin reductase activity and Cyb5R enzyme, a higher hemoglobin F ratio with a predisposition to oxidation, a more acidic gastric pH and a reducing intestinal flora. The clinical presentation depends on the level of methemoglobinemia and the patient's comorbidities. The presentation can be asymptomatic, cyanosis, dyspnea, non-specific symptoms (headache, photophobia, irritability, asthenia, lethargy), leading in severe cases to shock, respiratory depression, or neurological alteration (coma, convulsions), possibly leading to death. The diagnosis of methemoglobinemia is based on clinical manifestations and tests such as blood gas analysis, which allow quantification of methemoglobin levels in the blood. It should be suspected in cases of unexplained cyanosis or hypoxia that does not respond to oxygen therapy, with normal PaO2 and SatO2, in patients with a personal or family history of methemoglobinemia or who have being in contact with causal agents.1,2,6
The management of methemoglobinemia focuses on the identification and elimination of trigger factors, supportive care that may require admission to the intensive care unit, as well as specific treatment. The treatment of choice for methemoglobinemia is methylene blue, with some exceptions which will be explained later. Its mechanism of action involves the reduction of methemoglobin to functional hemoglobin by the transfer of electrons. These electrons are obtained from the oxidation of NADPH generated in the pentose monophosphate pathway.6,7 The mechanism of action of methylene blue is shown in Figure 1.12
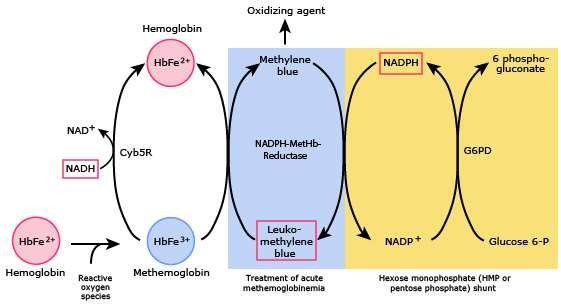
Figure 1 Modified from: Gregg XT, Prchal JT. Red blood cell enzymopathies. In: Hematology: Basic Principles and Practice, 7th ed, Hoffman R, Benz EJ Jr, Silberstein LE, et al (Eds), Elsevier 2017.
One exception is patients with a deficiency of glucose-6-phosphate dehydrogenase (G6PD), a crucial enzyme in the pentose phosphate pathway. Individuals affected by this deficiency have a reduced ability to generate NADPH, an enzyme essential for methylene blue to act as a reductant of methemoglobin. Furthermore, administration of methylene blue in these patients may trigger acute hemolysis.13 Therapeutic alternatives such as ascorbic acid may be considered in such patients. Finally, it should be noted that treatment strategies exist for severe cases, such as exchange transfusion and hyperbaric oxygen.14-16
In conclusion, methemoglobinemia represents a serious clinical condition characterized by elevated blood methemoglobin levels, defined as >1.5 g/dL (>5%). This condition can be congenital or acquired and can be triggered by a variety of factors, including exposure to foods rich in nitrates or nitrites, certain drugs, inadequately treated well water, and other chemicals. It is crucial to consider the possibility of methemoglobinemia in patients with cyanosis, with or without signs of hypoxia, who do not respond to supplemental oxygen, despite maintaining normal PaO2 and Sat O2 levels. The presence of very high methemoglobin levels (>50%) can be life-threatening. Administration of nitric oxide may increase the risk of methemoglobinemia due to its oxidizing power and nitrate production. Infants are particularly susceptible due to factors such as the presence of intestinal bacteria, gastric pH and enzyme activity.
The diagnosis of methemoglobinemia is made by blood gas analysis, which will reveal the presence of methemoglobin in the blood. The treatment of choice for methemoglobinemia is methylene blue. However, caution should be exercised in patients with glucose-6-phosphate dehydrogenase deficiency, as methylene blue is contraindicated in these cases. In such situations, ascorbic acid may be considered as a suitable alternative. Early identification and management of methemoglobinemia is essential to avoid serious and life-threatening complications.

©2024 Caballero-Estupiñán, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.