Journal of
eISSN: 2373-6437


Research Article Volume 13 Issue 2
Siracusa No. 51 Col. Lomas Estrella, Mexico City, Mexico
Correspondence: Hilda Adriana Castro-Rocha MD, Siracusa No. 51 Col. Lomas Estrella, Mexico City, Mexico , Tel 5514519200
Received: March 23, 2021 | Published: March 31, 2021
Citation: Hilda ACRMD. Safety and efficacy of Fibroquel® (polymerized type I collagen) in adult outpatients with moderate COVID-19: an open label study. J Anesth Crit Care Open Access. 2021;13(2):101‒108. DOI: 10.15406/jaccoa.2021.13.00478
Background: SARS-CoV-2 infection induces a hyperinflammatory syndrome, causing acute respiratory distress syndrome, and clinical deterioration. The current therapeutic investigation has been focusing on the development of novel immunosuppressant therapies to control cytokine production.
Objective: To evaluate the safety and clinical effect of intramuscular administration of polymerized type I collagen in adult outpatients with moderate COVID-19.
Design, setting, and participants: This was an open-label study that recruited 20 adult patients with confirmed COVID-19 diagnosis, from June 16, 2020 to September 20, 2020. The final date of follow-up was November 4, 2020.
Interventions: Patients received intramuscularly 1.5 ml of polymerized type I collagen (12.5 mg of collagen) every 12 h for 3 days and then every 24 h for 4 days.
Main outcomes and measures: The primary outcome was oxygen saturation >92% on room air without supplemental oxygen. The secondary outcome was the duration of symptoms.
Results: Of 20 patients who were recruited, 9 (45%) were male, with mean age of 49.5±11.2 years. Oxygen saturation >92% on room air without supplemental oxygen was achieved by 18 (90%) and 19 (95%) of patients at day 1 and 8 post-treatment with polymerized type I collagen (P=0.001). Most of the symptoms improved from day 3 of treatment concerning baseline. The neutrophil-to-lymphocyte ratio (NLR) and lactate dehydrogenase (LDH) (predictive biomarkers for moderate-severe COVID-19) decreased to statistically significant levels at day 8 post-treatment (P=0.023 and P=0.011, respectively). No serious adverse events were detected.
Conclusion and relevance: In the study more than 90% of the patients had mean oxygen saturation >92%. A shorter duration of the symptoms was determined. A significant decrease in NLR and LDH was also found. The study is limited by small sample size and short follow-up duration, and determination of clinical efficacy would require larger randomized trials with more definitive outcome measures.
Keywords: yoga, depression, holistic, anxiety, physical postures, sudarshan kriya yoga, imipramine, electroconvulsive therapy
As the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has spread throughout the globe, it has currently remained in Latin America. As of September 3rd, 2020, Mexico has become the seventh nation with confirmed cases and the third regarding SARS-CoV-2 related deaths worldwide, lagging only Brazil as the second country with the highest death toll worldwide.1 Most people with COVID-19 have only mild or uncomplicated symptoms, but approximately 10–15% of patients have moderate or severe disease that requires admission to hospital and oxygen support, and 3–5% require admission to an intensive care unit (ICU) mainly for ventilation assistance.2 The hallmark of the infection is viral pneumonia accompanied by host inflammation, pulmonary edema and a syndrome that resembles acute respiratory distress syndrome (ARDS).3 It results from exuberant activation of mononuclear cells that are related to an elevation of inflammatory soluble mediators, defective synthesis of type 1 interferons and decreased antiviral response. Pulmonary edema, lung failure, and multiple organ failure can lead to death.3,4 Clinical deterioration typically occurs during the second week of illness. Early studies of COVID-19 found that hospitalization most often occurs within 8 to 10.5 days of initially mild to moderate symptoms.2,5 Thus, there is an urgent need to find anti-inflammatory drugs to mitigate inflammatory organ injury in viral pneumonia. Some trials of immunomodulatory drugs are been performed,6 such as the RECOVERY trial, which has been demonstrating that in hospitalized patients with COVID-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation, or oxygen alone.7 However, nonhospitalized patients do not benefit from this therapy. For this reason, the search for therapeutic strategies that modulate inflammation and avoid, as far as possible, the long-term sequelae of the infection, is still fertile ground. A potential drug for immune modulation is the polymerized type I collagen. It is a g-irradiated mixture of pepsinized porcine type I collagen and polyvinylpyrrolidone in a citrate buffer solution. At 37ºC and neutral pH, the molecule does not form a gel, like a collagen, and its electrophoretic, physicochemical and pharmacological properties are modified by the covalent bond between the protein and the PVP.8 The polymerized type I collagen has been shown to induce negative regulation of pro-inflammatory cytokine production (IL-1β, TNF-α, IL-8, IL- 17, IFN-g, PDGF and TGF-β1),9-12 of the expression of adhesion molecules, ELAM-1, VCAM-1 and ICAM-1,10-12 of the enzyme cyclooxygenase (Cox) -110 through the modulation of the transcription factor NF-kB10,12,13 in inflamed tissues. Moreover, polymerized type I collagen has been shown to induce positive regulation of IL-10,12,13 and the presence of regulatory T cells.12,14 The systemic effect has been determined by intramuscular or subcutaneous administration of polymerized type I collagen to patients with active rheumatoid arthritis, as a therapeutic co-adjuvant with methotrexate (pilot and phase II studies), improving the count of swollen joints, morning stiffness, an ACR 50 in 57% of the patients and inducing remission in 30% of the patients. Polymerized type I collagen is safe and well-tolerated in long-term treatment, without adverse effects.15,16
This study was designed to evaluate the safety and efficacy of intramuscular administration of polymerized type I collagen in adult outpatients with moderate COVID-19.
Study design
This was an open-label study in adult outpatients with symptomatic COVID-19.
Participants
Patients were recruited from June 16, 2020, to September, 2020. All participants provided written informed consent. Trial candidates were identified in a medical appointment at their homes. The diagnosis was based on suggestive symptoms (fever, headache, cough or dyspnea, plus at least another symptom such as malaise, myalgias, arthralgias, rhinorrhea, throat pain, conjunctivitis, vomiting or diarrhea) and a positive real-time reverse-transcription polymerase chain reaction result for SARS-CoV-2.
Individuals were asked to provide information, including demography (data of birth, job, educational level, previous contact with infected individuals, date of onset of symptoms, etc.), preexisting conditions (including a history of hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease, hypertriglyceridemia, dyslipidemia, etc.) and symptoms. Exclusion criteria included hypersensitivity to polymerized type I collagen or any of its excipients, COVID-19 patients that required hospitalization, all pregnant or breast-feeding patients, patients with chronic kidney disease as determined by calculating an estimated glomerular filtration rate (eGFR), decompensated cirrhosis, congestive heart failure (New York Heart Association class III or IV), patients with cerebrovascular disease, autoimmune disease, cancer, multiorgan failure or immunocompromised (solid organ transplant recipient or donor, bone marrow transplant recipient, AIDS, or taking immunosuppressant biologic drugs or corticosteroids).
Study supplies were given to patients as a package and the study materials consisted of the medication, oxygen saturation monitor, and a symptom questionnaire booklet. Patients were instructed how to administer the study medication, to use the oxygen monitor and to complete de questionnaires.
Phone contact was attempted daily during the first 3 days of the trial to address participants’ questions, address any medication-related issues, and encourage assessment completion. Additional phone calls were conducted on a case-by-case basis when participant’s survey data indicated values outside the ranges. Patients were evaluated at 8 (1-day post administration of the last dose of the medication or placebo), and 15 days (8-days post administration of the last dose of the medication or placebo) by the physician.
Intervention
Participants received intramuscularly a dose of 1.5 ml of polymerized type I collagen (12.5 mg of collagen) every 12 h for 3 days and then every 24 h for 4 days. As concomitant therapy, the use of paracetamol and acetylsalicylic acid was only allowed.
Data collection
Epidemiologic, demographic, contact and exposure history, clinical presentations, chest CT, laboratory tests, treatment and outcome data were collected from inpatient medical records. Laboratory data collected for each patient included complete blood count, coagulation profile, serum biochemical tests (including renal and liver function, electrolytes, lactate dehydrogenase, D dimer and creatine kinase), serum ferritin and biomarkers of infection at baseline, 8 (1-day post administration of the last dose of the medication) and 15 days (8-days post administration of the last dose of the medication). Chest CT scans were done for all patients at baseline.17,18
Primary and secondary end points
The primary outcome was an oxygen saturation 92% or greater on room air without supplemental oxygen. For secondary endpoints, a reduction of symptoms, by at least 30% compared with baseline on a scale of symptom severity (0 = symptom is not present, 1 = mild, 2 = moderate and 3 = symptom is very severe) was considered as clinically relevant, and the number of hospitalizations.
The primary and secondary endpoints were measured using participants’ self-reported responses that were corroborated by the physician evaluation at 8- (1-day post administration of the last dose of the medication) and 15-days (8-days post administration of the last dose of the medication).
Adverse events and serious adverse events were recorded each day via physician and participant self-report.
Chest computed tomography (CT)
Radiological abnormalities on chest CT were evaluated by a semi-quantitative scoring system to estimate the pulmonary involvement.17,18
Statistical analysis
A descriptive analysis of the sample was performed. Continuous variables are expressed by mean and standard deviations (normal distribution), median and range, and proportions for categorical variables. The average differences between the groups represent the percentage of change post-treatment vs. pre-treatment. For continuous variables with normal distribution, the delta was calculated by taking the difference post vs. pretreatment over the pretreatment value. Mann-Whitney Rank Sum Test was performed to determine differences between groups for continuous variables. Statistical analyses were performed using the Sigma Stat 11.2 program (Aspire Software International, Leesburg, VA, USA). Data are P values less than or equal to 0.05 were considered significant.
Demographic and clinical characteristics
Twenty adult non-hospitalized patients with moderate COVID-19 were enrolled in the study. The mean (±SD) age of the patients was 49.5 ± 11.2 (range: 19-78) years. Nine patients (45%) of the population were male (Table 1). The most common coexisting conditions were diabetes in 5 (25%) patients, congestive heart failure and asthma in 4 (20%) patients. Seven (35%) patients were overweight and 2 (10%) were obese (Table 1). Pulmonary abnormalities were determined in 15 (75%) patients; of these 8 (40%) patients had less than 20% lung parenchymal involvement and 7 (35%) between 20-50% (Table 1). The most common symptoms at illness onset were myalgia (80%), arthralgia (75%), headache (70%), dyspnea (65%), cough (60%), throat and chest pain (55%), ageusia (50%) and anosmia (45%) (Table 1).
|
Characteristic |
All Subjects (N= 20) |
Day 1 post-treatment |
Day 8 post-treatment |
P-value |
|
Comparability of randomized groups |
||||
|
Age (years), mean ± SD Median Range |
49.5 ± 11.2 47.5 19.0 – 78.0 |
|||
|
Sex, Male, n, (%) |
9 (45) |
|
|
|
|
BMI (kg/m^2), mean ± SD Median Range |
25.97 ± 4.45 25.76 19.53 – 38.01 |
|
|
|
|
Overweight, n, (%) Obesity, n, (%) |
7 (35) 2 (10) |
|
|
|
|
Baseline Chest CT Score 0% <20% ≥20% |
5 (25) 8 (40) 7 (35) |
|
|
|
|
Days from diagnosis to onset of treatment), mean ± SD Median Range |
5.94 ± 3.97 6.00 0.00 – 12.00 |
|
|
|
|
Oxygen Saturation |
|
|
|
|
|
pSO2<92% (%)
|
8 (40)
|
2 (10)
|
1 (5) |
0.001 |
|
pSO2; mean ± SD Median range |
88.95 ± 8.54 92.0 63-97 |
94.53 ± 1.84 95 91-98 |
95.59 ± 2.09 96 91-98 |
0.002a <0.001b |
|
Laboratory variables |
|
|
|
|
|
Complete blood count |
|
|
|
|
|
Leukocyte count (x10^3/µL), mean ± SD Median Range |
8.26 ± 3.85 6.90 3.80 – 16.00 |
6.59 ± 1.97 6.44 3.98 – 10.36 |
6.24 ± 1.31 6.24 4.49 – 9.04 |
0.288a 0.273b |
|
Hemoglobin (g/dL), mean ± SD Median Range |
15.54 ± 1.33 15.55 12.70 – 17.90 |
14.70 ± 1.57 15.05 12.00 – 17.50 |
14.78 ± 1.36 14.7 12.60 – 17.30 |
0.206a 0.125b |
|
Platelets (K/µL), mean ± SD Median Range |
244.53 ± 67.84 250.00 142 - 418 |
392.38 ± 134.57 331.00 235 - 750 |
299.60 ± 78.44 279.00 180 - 427 |
<0.001a 0.041b |
|
Lymphocyte count (%), mean ± SD Median Range |
27.94 ± 14.53 26.00 4.00 – 58.10 |
32.36 ± 9.48 33.5 14.00 – 47.10 |
38.33 ± 6.42 37.35 28.00 – 52.10 |
0.358a 0.041b |
|
Neutrophil count (%), mean ± SD Median Range |
64.94 ± 14.00 61.00 45.80 – 88.20 |
55.86 ± 10.16 55.25 40.50 – 73.00 |
49.21 ± 6.35 50.20 34.10 – 64.00 |
0.041a <0.001b |
|
Neutrophil-lymphocyte ratio (NLR), mean ± SD Median Range |
4.14 ± 5.10 2.35 1.00 – 22.00 |
2.01 ± 1.19 1.56 0.86 – 5.21 |
1.36 ± 0.41 1.33 0.65 – 2.29 |
0.141a 0.023b |
|
Liver function test (LFT) |
|
|
|
|
|
Total bilirubin (mg/dL), mean ± SD Median Range |
0.62 ± 0.41 0.46 0.28 - 1.86 |
0.64 ± 0.35 0.50 0.31 - 1.52 |
0.59 ± 0.37 0.46 0.23 - 1.65 |
0.645a 0.707b |
|
Direct bilirubin (mg/dL), mean ± SD Median Range |
0.22 ± 0.11 0.18 0.10 - 0.45 |
0.21 ± 0.09 0.19 0.09 - 0.36 |
0.19 ± 0.08 0.17 0.09 - 0.40 |
0.908 a 0.540 b |
|
Indirect bilirubin (mg/dL), mean ± SD Median Range |
0.40 ± 0.32 0.30 0.16 - 1.41 |
0.43 ± 0.27 0.43 0.21 - 1.16 |
0.40 ± 0.30 0.30 0.13 - 1.25 |
0.548 a 0.937 b |
|
Aminotransferase, serum aspartate (AST) (U/L), mean ± SD Median Range |
38.10 ± 20.19 34.50 12.80 – 86.00 |
48.86 ± 36.72 42.10 12.13 – 141.00 |
24.11 ± 9.90 23.10 4.00 -45.20 |
0.129 a 0.314 b |
|
Aminotransferase, serum alanine (ALT) (U/L), mean ± SD Median Range |
41.95 ± 22.22 40.90 13.00 – 81.00 |
60.61 ± 41.93 52.90 11.7 – 157.00 |
34.70 ± 16.54 33.70 15.10 – 68.00 |
0.607 a 0.016 b
|
|
Albumin (g/dL), Mean ± SD Median Range |
4.11 ± 0.26 4.09 3.77-4.70 |
4.16 ± 0.43 3.98 3.50-4.76 |
4.29 ± 0.43 4.27 3.50-5.30 |
0.903 a 0.194 b |
|
Fasting glucose (mg/dL) Mean ± SD Median Range |
138.98 ± 104.00 101.00 80.00 – 446.00 |
103.85 ± 19.90 98.00 81.00 - 147.30 |
97.81 ± 25.14 92.00 79.40 – 185.00 |
0.716 a 0.056 b |
|
Lactate dehydrogenase (LDH) (U/L) Mean ± SD Median Range |
282.31 ± 110.47 266.30 125.00 – 505.00 |
248.21 ± 93.44 220.00 180.00 – 560.00 |
191.99 ± 38.43 198.00 135.10 – 268.00 |
0.234 a 0.011 b |
|
Ferritin (ng/mL) Mean ± SD Median Range |
728.46 ± 979.73 317.50 19.80 – 3411.00 |
610.16 ± 606.61 379.00 24.00 – 1896.00 |
363.12 ± 340.38 287.00 21.00 – 1165.88 |
0.953 a 0.462 b |
|
D-dimer (ng/dL) Mean ± SD Median Range |
500.45 ± 541.72 320.00 0.93-2164.40 |
945.84 ± 753.41 630.00 0.87-2170 |
627.01 ± 740.73 350 0.69-2840 |
0.054 a 0.583 b |
|
Summary of Comorbidities |
|
|
|
|
|
None, n, (%) |
10 (50) |
|
|
|
|
One, n, (%) |
7 (35) |
|
|
|
|
2 or More, n, (%) |
3 (15) |
|
|
|
|
Clinical Comorbidities |
|
|
|
|
|
Hypertension, n, (%) |
2 (10) |
|
|
|
|
Hypertriglyceridemia, n, (%) |
2 (10) |
|
|
|
|
Diabetes, n, (%) |
5 (25) |
|
|
|
|
Congestive heart failure, n, (%) |
4 (20) |
|
|
|
|
Chronic respiratory disease (emphysema), n, (%) |
2 (2.3) |
|
|
|
|
Asthma, n, (%) |
4 (4.5) |
|
|
|
|
Chronic liver disease (chronic hepatitis, cirrhosis), n (%) |
0 (0.0) |
|
|
|
|
Chronic kidney disease, n, (%) |
1 (5) |
|
|
|
|
Cancer, n (%) |
0 (0.0) |
|
|
|
|
Immune deficiency (acquired or innate), n, (%) |
0 (0.0) |
|
|
|
|
Symptoms |
|
|
|
|
|
Dyspnea, n (%) Δ (%) |
13 (65) |
4 (20) 69 |
1 (5) 92 |
|
|
Cough, n (%) Δ (%) |
12 (60) |
9 (45) 25 |
5 (25) 58 |
|
|
Chest pain, n (%) Δ (%) |
11 (55) |
6 (30) 45 |
3 (15) 73 |
|
|
Headache, n (%) Δ (%) |
14 (70) |
8 (40) 43 |
4 (20) 71 |
|
|
Sore throat, n (%) Δ (%) |
11 (55) |
3 (15) 73 |
1 (5) 91 |
|
|
Arthralgia, n (%) Δ (%) |
15 (75) |
5 (25) 67 |
0 (0) 100 |
|
|
Myalgia, n (%) Δ (%) |
16 (80) |
7 (35) 56 |
1 (5) 94 |
|
|
Ageusia, n (%) Δ (%) |
10 (50) |
5 (25) 50 |
2 (10) 80 |
|
|
Anosmia, n (%) Δ (%) |
9 (45) |
5 (25) 44 |
1 (5) 89 |
|
|
Diarrhea, n (%) Δ (%) |
4 (20) |
4 (20) 0 |
2 (10) 50 |
|
|
Abdominal pain, n (%) Δ (%) |
6 (30) |
3 (15) 50 |
2 (10) 67 |
|
Table 1 Baseline demographic and clinical characteristics of the trial population and study endpoints
a:baseline vs. day 1 post-treatment; b:baseline vs. day 8 post-treatment. Δ: Delta calculated by taking: [(Baseline data - Day 1, or 8 of follow-up) / Baseline]*100. pSO2; oxygen saturation, SD; standard deviation. Bold numbers: significant difference
Primary outcome
Mean oxygen saturation readings at baseline were 88.95 ± 8.54 (range: 63 – 97; Figure 1; Table 1). At days 1, and 8 post-treatment, mean oxygen saturation readings were 94.53 ± 1.84 (range: 91- 98; P=0.002; Figure 2A) and 95.59 ± 2.09 (range: 91 – 98; P<0.001; Figure 2A); and the percentage of subjects with oxygen saturation readings ≥92% were 90% and 95%, respectively (P=0.001; Figure 1; Table 1). It should be noted that, at day 3 of treatment with polymerized type I collagen was determined the first statistically significant increase of oxygen saturation readings (Figure 2A).
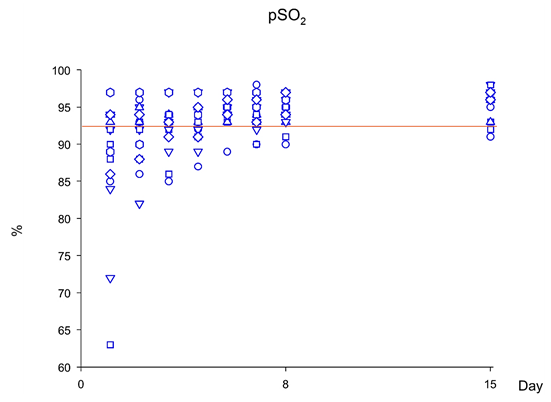
Figure 1 Oxygen saturation while breathing ambient air during the study in patients with moderate COVID-19 treated with polymerized type I collagen. Orange line: pSO2=92%.

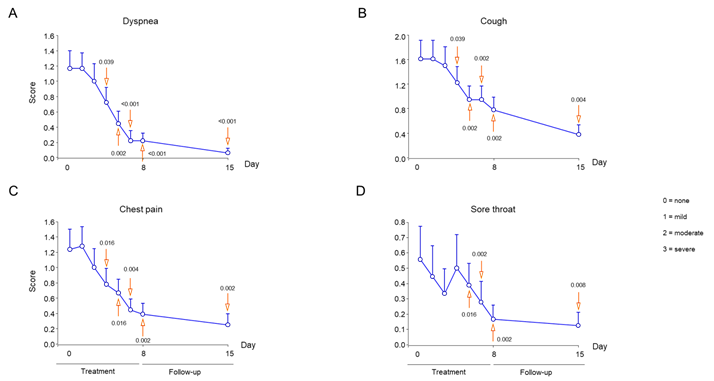
Figure 2 Vital signs in patients with moderate COVID-19 treated with polymerized type I collagen. (A) Oxygen saturation while breathing on room air without supplemental oxygen, (B) Temperature, (C) Hearth rate, and (D) Respiratory rate.
Secondary outcomes
Symptom improvement was evaluated every day and compared with baseline. Temperature decreased at statistically significant levels during day 3 of treatment (Figure 2B), while respiratory and heart rates at days 4 and 5, respectively (Figure 2C and D). At days 1 and 8 post-treatment with polymerized type I collagen, 69% and 92% of patients no longer had dyspnea vs baseline, respectively (Table 1). Compared with baseline significant improvements in dyspnea intensity were determined at day 2 of treatment (Figure 3A). The intensity of the symptoms such as cough, chest pain, sore throat, headache, arthralgia, myalgia, anosmia and ageusia had a significantly improved during the week of treatment with polymerized type I collagen (Figure 3).
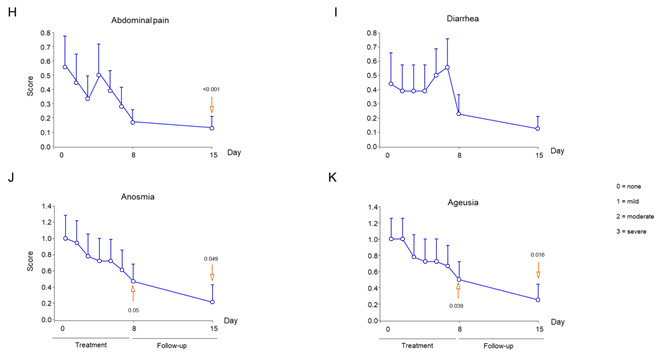
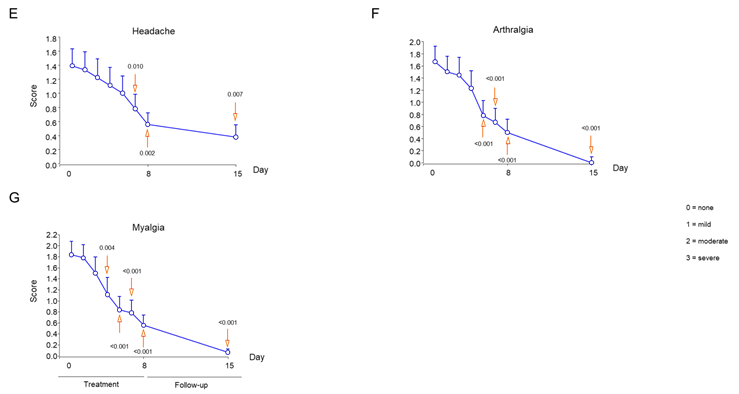
Figure 3 Intensity of symptoms during treatment and follow-up of outpatients with symptomatic COVID-19 treated with polymerized type I collagen or placebo. (A) Dyspnea, (B) cough, (C) chest pain, (D) sore throat, (E) headache, (F) arthralgia, (G) myalgia, (H) abdominal pain, (I) diarrhea, (J) anosmia, and (K) ageusia. The intensity of the symptom was evaluated on a 4-point rating scale (0 = without symptom, 1 = mild, 2 = moderate, 3 = severe). Blue lines represent the group of patients under polymerized type I collagen treatment (n=20). Results depict mean ± standard deviation. Orange arrows show the day in which the treatment reached a P < 0.05 compared to baseline for polymerized type I collagen treatment.
Invasive mechanical ventilation, supplemental oxygen, hospitalizations and deaths
Two patients received oxygen without invasive mechanical ventilation (via nasal cannula) during 1 week, one patient for 1.5 weeks, 3 patients for 2 weeks, and 1 patient for 2.5 weeks (1.5-10 L/min). All patients were alive and no deaths occurred. No subjects in the polymerized type I Collagen treatment group were hospitalized.
Laboratory assay
No differences in laboratory results were found at baseline vs day 1 and 8 post treatment (Table 1). Nonetheless, at day 8 post-treatment lymphocyte count increased at statistically significant levels vs baseline (P=0.041; Table 1), while neutrophil count decreased at day 1 and 8 post-treatment vs baseline (P=0.041 and P<0.001, respectively; Table 1). Neutrophil-to-lymphocyte ratio (NLR) decrease at day 1 post-treatment compared to baseline (P=0.023; Table 1; Figure 4A). At day 1 post-treatment, lactate dehydrogenase (LDH) levels decreased at statistically significant levels (P=0.011; Table 1; Figure 4B).

Figure 4 Neutrophil to lymphocyte ratio and lactate dehydrogenase during treatment and follow-up of outpatients with moderate COVID-19 treated with polymerized type I collagen. (A) Neutrophil to lymphocyte ratio (NLR), and (B) lactate dehydrogenase. Blue lines represent the group of patients under polymerized type I collagen treatment (n=20). Results depict mean ± error standard of the mean. Orange arrows show P-value (1-day or 8 days post-treatment compared to baseline).
Transverse chest computed tomograms
Imaging of patients with symptomatic moderate COVID-19 revealed characteristic patchy infiltration, progressing to large ground-glass opacities that often present bilaterally. Abnormalities on chest computed tomograms or radiographs was detected among 75% of the patients collected in this study (Table 1).
Medication at baseline
Of 20 patients at baseline, 5 (25%) were being treated with acetaminophen, 5 (25%) with acetylsalicylic acid, 1 (5%) with antivirals (oseltamivir), 7 (35%) with antibiotics (azithromycin, ceftriaxone, penicillin, clarithromycin and levofloxacin), 4 (20%) with anticoagulants and 3 (15%) with dexamethasone. However, when patients began the treatment with polymerized type I collagen, dexamethasone and antibiotics was suspended.
Adverse events
In the study, no serious adverse events were detected. Polymerized type I collagen was safe and well-tolerated during the study. Patients had pain at the application site lasting less than 15 to 20 min.
This study demonstrated that outpatients with moderate COVID-19 treated with intramuscular polymerized type I collagen had an increment in mean oxygen saturation readings on room air without supplemental oxygen, as previously reported by Carpio-Orantes et al.19 Polymerized type I collagen also shortened symptom duration.
It is important to mention that the treatment with polymerized type I collagen decreased NLR. It is a simple biomarker of inflammation that can be measured during routine hematology. Previous studies have exhibited that higher NLR is associated with clinical deterioration and mortality for COVID-19 patients.20 Thus, a decrease in NLR suggests a better outcome for patients treated with polymerized type I collagen. On the other hand, the decrease in the neutrophil count and the increase in the lymphocyte count, contributes to decreasing the systemic inflammation, which suggests that the polymerized type I collagen exerts a down-regulation of inflammation.
Besides, LDH is an enzyme involved in energy production and that is found in almost all cells in the body. Tests that measure the concentration of LDH in the blood are commonly used to monitor tissue damage associated with a wide range of disorders, including liver disease and interstitial lung disease. The increase of LDH reflects tissue/cell destruction and is regarded as a common sign of tissue/cell damage, suggesting viral infection or lung damage, such as pneumonia induced by SARS-CoV-2. We also know that serum LDH has been identified as an important biomarker for the activity and severity of idiopathic pulmonary fibrosis. More specifically, in patients with the severe pulmonary interstitial disease, the increase of LDH is significant and is one of the most important prognostic markers of lung injury. There is convincing evidence linking high LDH levels in critically ill patients, through COVID-19, with increasing activity and the extent of lung injury.2 Thus, a statistically significant decrease of serum LDH levels in patients treated with polymerized type I collagen suggests a down-regulator mechanism of inflammation.
Based on the decrease of NLR and LDH in patients under treatment with polymerized type I collagen, we infer that the medication fits with the profile of anti-inflammatory drugs, such as dexamethasone, colchicine, and Janus kinase inhibitors.21-24 Nonetheless, in contrast to dexamethasone, which is an immunosuppressor, polymerized type I collagen is a down-regulator of inflammation. For this reason, we suggest that polymerized type I collagen does not affect the functions of T and B cells, as previously reported 15 and, in consequence, would not be expected to affect serum viral load or the risk of acquiring other infectious agents, water and salt retention, blood pressure, glycemia, muscle weakness, gastrointestinal bleeding and psychological disturbances, as observed with corticosteroids25 although this must be confirmed in further studies. Moreover, polymerized type I collagen was safe and well-tolerated. It did not induce liver damage, impairment of hematopoiesis, alterations in blood count, as was seen in Table 1.
The majority of COVID-19 patients show benign disease courses where patients overcome viral inflammation by robust but not overreactive immune response.2 Thus, few studies have been focused on the treatment of this vulnerable population. To avoid complications in medical care, visits to the emergency department and in the worst case, hospitalizations, we suggest that the early treatment with polymerized type I collagen could prevent patients from deterioration because of hyperinflammation, elongation of the symptom’s duration and the possible sequelae. The potential advantages of polymerized type I collagen for symptomatic outpatients’ treatment of COVID-19 include its safety, low cost, and intramuscular administration.
This study has several limitations. First, it was an open small study and it was conducted within a single geographic area, so these findings should be regarded as preliminary. The study needs to be replicated in larger trials with a more heterogeneous study population. Second, there was a small number of endpoint events, which makes the findings fragile. The follow-up duration was short and did not measure the effect of polymerized type I collagen on persistent symptoms or late deterioration. We did not collect information on virologic measures to prove that polymerized type I collagen does not increase viral load. The possible usefulness in the treatment of hospitalized and/or invasive ventilation patients is still unknown. Finally, if polymerized type I collagen is determined to be effective in treating symptomatic COVID-19, the underlying mechanisms need further clarification.
Summing up, in this open-label study of adult outpatients with moderate COVID-19, polymerized type I collagen was safe and had clinical efficacy for the treatment of hypoxemic COVID-19 patients.
The author declares no competing interests.

©2021 Hilda. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.