Journal of
eISSN: 2373-6437


Case Report Volume 2 Issue 5
1Department of Anaesthesiology & Intensive care, India
2Sr. Consultant Physician & Cardiologist, India
3Intensivist, India
Correspondence: Sanjay Prakash, Consultant, Anaesthesiology & Intensive care, Orchid Hospital, AG1/174-D, Vikaspuri, New Delhi, India, Tel +918285214546
Received: June 23, 2015 | Published: July 27, 2015
Citation: Prakash S, Gupta HO, Wankhade PR, Khatri R (2015) Rare Survival in a Case of Aluminium Phosphide Poisoning. J Anesth Crit Care Open Access 2(5): 00068. DOI: 10.15406/jaccoa.2015.02.00068
Aluminium Phospide (ALP) is a cheap, commonly used rodenticide and pesticide in India, which is available easily by various names like Celphos. Unfortunately, its high toxicity and unavailability of specific antidote results in very high mortality after ingestion. Treatment is largely supportive, which includes gastric lavage, correction of acidosis, inotropes and ventilator support. The outcome is poor, largely due to delays in appropriate aggressive management and doubtfulness in the minds of clinicians regarding results in aluminium phosphide poisoning patients. We present here a case of aluminium phosphide poisoning who survived even after ingesting a toxic dose of Celphos tablet because of apt aggressive management at the appropriate time.
Keywords: aluminium phosphide (alp), phosphine gas (PH3), electron transport chain (ETC)
SpO2 oxygen saturation; ABG, arterial blood gas; ROS, reactive oxygen species; NT-proBNP, N-terminal pro-brain natriuretic peptide; LVF, left ventricular failure
Aluminium Phosphide is a solid type of fumigant which is available in tablet and powder form. In one study it was found to be the most common cause of acute poisoning in India.1 As the Lethal Dose of Aluminium Phosphide is 150-500 mg for an adult even a single tablet of 3 gm can cause mortality.2 It is colourless and odourless, however on exposure to air it gives a foul odour (garlicky or decaying fish) due to the presence of substituted phosphines and diphosphines.3 This odour also helps in the diagnosis of ALP poisoning. ALP after exposure to moisture or acid in the stomach releases the implicating phosphine PH3 gas.
AIP+3H2O=AL(OH)3+PH3 (In air)
AIP+ 3HCL =ALCL3+PH3(in air and stomach)
Exact mechanism of toxicity is still not fully understood, but it is speculated that it acts by blocking the cytochrome c oxidase in the mitochondria, thus disrupting the Oxygen ( ) transport in Electron Transport Chain (ETC) eventually leading to cell death.4 A mitochondrion has double membrane. The outer membrane is permeable but the mitochondrial inner membrane (MIM) is highly impermeable. This ensures the controlled passage of molecules across the MIM between Intermembrane space and central Mitochondrial Matrix and a relaxed exchange between Intermembrane space and cytoplasm. The mitochondrial membrane potential is maintained by the Electron Transport Chain (ETC) which is embedded within Mitochondrial Inner Membrane.
ETC receives high energy electrons predominantly from
The electrons are transported from NADH to O2 through a chain of three large protein complexes (Figure 1): Complex I (NADH-Q, Oxidoreductase)
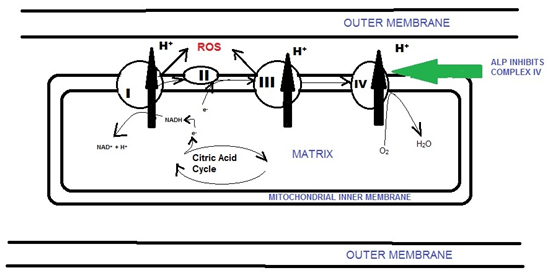
Figure 1 Electron transport chain in the Mitochondria showing site of action of ALP and production of ROS.
At complex IV electrons are transferred to molecular oxygen, to generate H2O. Phosphine primarily inhibits cytochrome c oxidase, or Complex IV of the ETC, thus leading to the inability of mitochondria to utilize O2 and causing cellular hypoxia.5 There occurs generation of reactive oxygen species (ROS), mainly Superoxide and Hydrogen peroxide causing cellular oxidative stress. The primary source of ROS is the Mitochondrial ETC.6 These ROS are highly damaging to biological macromolecules, ultimately leading to cell death. If the flow of electrons is impeded production of superoxide O2- can become very significant.7 A detailed analysis of the types of ROS induced by Phosphine and the roles of specific protectants identified hydrogen peroxide H2O2 as the most significant ROS and glutathione as the strongest protective antioxidant.8 Glutathione not only protected against oxidative cell damage, but enhanced cell survival as well. Magnesium ions help in scavenging free radicals by raising Glutathione level, also Magnesium is an anti arrhythmic agent. It has been shown that antioxidants, most notably melatonin, can prevent most of the oxidative damage induced in a range of tissues in rat and that melatonin can protectively maintain the levels of glutathione.9 Most patients succumb to its toxicity because of unavailability of specific antidote and also because of the delay in initiation of proper treatment.
A 45years male, was received in ICU two hours after alleged deliberate ingestion of a 3gm tablet of ALP. He presented with restlessness and agitation. There was a garlicky smell coming from his mouth. His relatives also provided the packet of tablets bearing the name of content as aluminium phosphide. The heart rate was 120 pm, peripheral pulses were feeble, blood pressure was not recordable and patient had respiratory distress. ABG showed metabolic acidosis. Inotropes (dopamine & nordrenaline) were started. A diagnosis of ALP poisoning was made based on his symptoms and the packet provided by relatives. Stomach wash was performed. Blood pressure increased gradually. SpO2 remained normal on Hudson mask with a high respiratory rate. A CVP and arterial line were secured. Intravenous magnesium was started and soda bicarbonate was given as bolus and an infusion was also started. ABG was done serially to correct acidosis. Blood pressure remained normal on high dose inotropes. After a few hours he had ventricular fibrillation with seizures. Chest compression was started, DC Shock 360 Joules given while he was intubated and put on a ventilator. After 15minutes of CPR, patient was revived. One hour after revival patient again had cardiac arrest. He got revived again after ten minutes of CPR.
Grave prognosis was again explained to relatives, but intensive treatment was continued. After thirty minutes BP came up gradually. His Kidney and Liver functions continued to be normal. The next day inotropes were still going in high doses, SpO2 was 95% on a of 60%, the kidney function report was normal while liver function showed little derangement (SGOT-126 and SGPT-82). On the fourth day his vitals were normal with low dose inotropes. SpO2 was >95% on a FiO2 of 40%. He was conscious and alert. Chest X-ray showed small opacities bilaterally. As he was better clinically extubation was done. Ten hours, post extubation his respiratory distress increased. Chest X-ray showed increased opacities bilaterally. Plasma NT-proBNP levels were elevated (6134). Echocardiography showed Regional wall motion abnormality with ejection fraction- 44%. The patient was in LVF and was given Injection frusemide followed by reintubation and ventilation. Meanwhile, his vitals remained stable on low dose inotropes. After a few days of being re-ventilated, he improved, X-ray had cleared gradually (Figure 2). The patient was extubated and subsequently shifted out of ICU on chest and limb physiotherapy.
The patient presented with a chief complaint of restlessness along with shock. The garlic smell and the packet of tablets established the diagnosis of ALP Poisoning. Later he had an episode of seizures also. Seizures are known to occur in ALP poisoning because of the suppression of acetylcholine esterase by phosphine which increases acetylcholine neurotransmission and in extreme cases can cause excitotoxicity and seizures.10 Acute cardiovascular collapse is the most common presentation seen in 60% to 100% of cases and also in our patient.11 This is secondary to the direct effect of PH3 on cardiac myocytes, fluid loss, and adrenal gland damage.12 PH3 inhibits the protein complex IV or the cytochrome c which is responsible for the final uptake of O2 in the electron transport chain (ETC) and converts it to water with H+. Thus its inhibition leads to inability of cell to O2 use and eventually cell death. The patient was given supportive treatment as there is no specific antidote available for ALP. Early use of soda bicarbonate and magnesium may have helped in saving the patient. Magnesium ions help in scavenging free radicals by raising Glutathione level, also magnesium is an anti arrhythmic agent which helped the patient. This patient also had myocardial damage and developed LVF as evident clinically and by increased plasma NT-proBNP levels. PH3 is known to cause myocardial damage and LVF can be attributed to its direct effect on myocardium.
This patient could be saved because of early initiation of proper treatment, Invasive monitoring and timely therapy with magnesium and soda bicarbonate infusion. Still, there is a lot of scope in the research of ALP as exact mechanism is not yet fully understood, but as per the discussion above further human studies need to be undertaken to find new modalities of treatment like- use of melatonin and glutathione supplements in ALP Poisoning. Finally, we should leave any presumptions regarding outcomes in ALP poisoning and continue the treatment aggressively.
None.
Author declares there are no conflicts of interest.
None.

©2015 Prakash, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.