Journal of
eISSN: 2373-6437


Research Article Volume 13 Issue 2
University of Arizona, USA
Correspondence: Craig Weinkauf, MD, PhD, University of Arizona, 1501 N. Campbell Ave, Room 4410,Vascular Surgery, Tucson, Arizona, USA 85724, Tel 443-205-0728
Received: March 22, 2021 | Published: March 29, 2021
Citation: Saha S, Bazzell M, Dull R, et al. Protective ventilation measures improve outcomes during major vascular surgery. J Anesth Crit Care Open Access. 2021;13(2):93‒99. DOI: 10.15406/jaccoa.2021.13.00477
Background: In major endovascular and open vascular surgery cases, pulmonary complications remain persistently high and the most prevalent. Despite strong evidence from intensive care unit (ICU) practices demonstrating benefits of ventilation management with low tidal volume and high positive end expiratory pressure (PEEP), no consensus exists regarding protective ventilation use intraoperatively.
Methods: A single institute, patient and surgeon blinded, prospective, randomized study design was used. Patients undergoing major vascular surgery (vascular surgery scheduled for >120 minutes and requiring general anesthesia) from 2015-2016 were randomized to pre-defined control (n = 14) or intervention (n =19) intraoperative ventilation arms. As described later, intervention consisted of a combination of low tidal volume, optimized positive end expiratory pressure (PEEP) and low intraoperative FiO2. Primary outcomes included all-cause mortality, myocardial infarction (MI) and reintubation within 7 post-operative days (POD). Secondary outcomes included atelectasis, pulmonary function measures, hospital length of stay and post-operative complications of re-intubation, pneumonia, sespsis, unplanned readmission or return to operating room, and/or mortality.
Results: The intervention arm had significantly reduced post-operative atelectasis ((p <0.02) and increased post-operative SpO2 (p< 0.02). The intervention arm also had a significantly lower length of hospital stay (6.9±5.5 vs 3.3±1.8, p < 0.016). This was corroborated by a multivariate regression analysis that showed therapy was independently correlated with decreased length of stay (p<0.007).
Conclusion: Our data indicate a combination of low tidal volumes, optimized PEEP and low FiO2 improves outcomes of patients undergoing major vascular surgery. Importantly, our study demonstrates that these study parameters for evaluation of intraoperative ventilation management are feasible in a busy academic center and a larger clinical trial is worthy. Protective intraoperative ventilation measures could have significant effects on vascular surgery outcomes.
The idea of lung protective has been utilized in ICU care for many years, but it is not widely adopted in intraoperative care. This is in part because studies have used various ventilation strategies and had mixed results in outcomes.1-6 Lung protective ventilation originated in critical care medicine with the ARDS net trial in 2000.7 The ARDS net study was multicenter randomized trial that showed mechanical ventilation with lower tidal volumes rather than traditional higher volumes, decreased mortality and number of days of ventilator use. These and related data have shaped ICU ventilator management and have started to influence intraoperative management.
Similar to other practices, anesthesia practices at our institution regarding intra-operative ventilation vary based on anesthesiologist preference; however, they typically include the regular use of a PEEP of 3-5 mmHg, tidal volumes of 450-650 ml (not calculated based on predicted body weight), and FiO2 of 80% or greater.8,9 Intra-operative ventilation management at our institution is similar to typical ventilation strategies for most nationwide anesthesiologists, with a trend towards lower tidal volume ventilation and routine use of PEEP.5,10-13 A recent multi-center observational study found in 3,000 patients in 49 hospitals, the mean intra-operative tidal volume to be 7.7 ml/kg; 18% of patients received > 10 ml/kg Vt and there was minimal use of recruitment maneuver.9
Several worldwide multicenter randomized controlled trials studied methods of lung protective ventilation in the abdominal surgery setting.3,4,6,11-15 The PROVAR trial was a 50 patient randomized control trial (RCT) study examining variable versus conventional ventilation with similar PEEP and FiO2.3 In this study, there was no difference in intraoperative or postoperative respiratory function between both groups. The iPROVE trial was a similar but larger RCT that examined open lung ventilation as well as standard postoperative CPAP or oxygen therapy after abdominal surgery.4 Despite the multiple varying methods of intraoperative ventilation and postoperative CPAP/O2, the study failed to show any change in postoperative complications. The PROVHILO trial had patients randomly allocated either high PEEP of 12 or low PEEP of 2 at tidal volumes of 8ml/kg.12 It failed to show any protective benefit in the open abdominal surgery population, but showed the higher PEEP group was more likely to develop intra-operative hypotension. The above trials have examined variable ventilation, open lung ventilation, standard postoperative CPAP or oxygen therapy, and high versus low intraoperative PEEP. In contrast to these trails, the IMPROVE found a statistical difference in the length of hospital stay and reintubation rate with intraoperative ventilation using tidal volume of 6-8ml/kg, PEEP of 6-8 cm of water and recruitment maneuvers every 30 minutes.14
No similar trials evaluating protective strategies for intraoperative ventilation have been conducted in vascular surgery patients, despite their high pulmonary risk. Post-operative pulmonary complications, including pneumonia and re-intubation, are a key source of morbidity and mortality in patients undergoing major vascular surgical procedures. Compared to other surgical patients, vascular patients are at particular risk for pulmonary complications because of their increased frequency of advanced age, tobacco use, COPD and poor mobility (pre-operative and post-operative). Several intraoperative risk factors are also encountered during major vascular surgery including blood loss, fluid shifts and ischemia-reperfusion organ injury, which can all foster conditions for post-operative pulmonary complications. Despite these risks, there are currently no other studies examining lung protective ventilation strategies in the vascular surgery setting. In addition, our study design using a combination of PEEP optimized for each individual patient and low FiO2 in addition to low tidal volumes based on weight is unique in the field.
This was designed as a single institution, patient and surgeon blinded, randomized, controlled trial with patients enrolled between 2015 and 2016. Patients were included if they were scheduled to undergo a major vascular surgery, which was defined as operations scheduled for >120 minutes of operative time, >48 hours for planned hospital stay and needing general anesthesia with endotracheal intubation. Exclusion criteria included age younger than 18 years, patients with a history of progressive neuromuscular illness, and mechanically ventilation, respiratory failure or sepsis in prior two weeks.
Study participants were randomized into one of two study arms. In our control group, the anesthesiologist gave intubated patients tidal volumes of 600-650cc for males, 500-550cc for females. No recruitment maneuvers were performed, unless patient was hypoxic. PEEP was maintained between 3-5 mm Hg, FiO2 greater than or equal to 70%. These parameters were picked to mimic the average anesthesia practice at the time. The intervention group differed in three key ways, 1) Low tidal volumes: The intervention arm was given tidal volumes calculated 6 ml/kg based on ideal body weight. Ideal body weight was calculated based on the Robinson formula which takes into account the patient’s height. 2) Optimized PEEP: Each patient was given optimal PEEP, which was determined through the use of lowest inflection point on the pressure-volume curve or a maximum PEEP of 12 if the lower inflection point was greater than 12. Neuromuscular blockers were concurrently used for optimal PEEP determination. 3) Low inspired oxygen concentrations: Inspired oxygen concentrations, less than or equal to FiO2 of 35%, or higher as needed to maintain SpO2 equal to or greater than 95%. Other anesthetic decisions regarding intra-operative care, use of fluids, antibiotics, pain management would be up to the discretion of the anesthesiology and surgical teams.
Outcomes were planned and defined before any patients were enrolled in the study. Primary end-points measured were all cause mortality, myocardial infarction and reintubation for any reason within 7 postoperative days. Secondary endpoints included atelectasis, peak flow, oxygen saturation, sepsis, hospital length of stay, ICU length of stay, unplanned readmission, unplanned return to operative room, use of non-invasive ventilation with the exception of patients with prior diagnosis of OSA who use CPAP at home.
Frailty Index was calculated using the modified 5 factor frailty index, which is based on the presence of the 5 co-morbidities: congestive heart failure within 30 days prior to surgery, insulin-dependent or noninsulin-dependent diabetes mellitus, chronic obstructive pulmonary disease or pneumonia, partially dependent or totally dependent functional health status at time of surgery, and hypertension requiring medication.16 Sepsis was defined by the third international consensus guidelines in 2016, that state a life threatening organ dysfunction caused by dysregulated host response to infection, including SOFA score>2.17 Pneumonia was defined by the Chest guidelines. Atelectasis was evaluated by obtaining pre-operative and post-operative chest X-rays, which were scored by blinded radiologists following the radiological atelectasis scoring system outlined in Parke et. al., 2014 in which a score of 0 was a normal chest x-ray, 1 is minor, 2 is moderate and 3 is severe atelectasis.18 Peak flow was quantified by a best of three testing of patients using peak flow meter pre-operative and on each day post-operatively. Oxygen saturation was measured post-operatively by turning oxygen (administered by nasal cannula) off (if tolerated) for 5 minutes and recording SpO2.
Wilcoxon sum analysis was performed to confirm the operative group differences between the control and intervention groups. It was also used to assess the respiratory outcome of atelectasis, and the clinical outcomes, including complications such as re-intubation, pneumonia, sepsis, mortality, unplanned readmission or return to OR and total amount of complications. Mean and standard deviation are reported for respiratory outcomes of peak flow, oxygen saturation, length of stay and ICU length of stay, using a two-tailed t-test to measure significance. Given the total n =30, only three variables were able to be chosen as predictor parameters for the multivariate analysis without risk of overfitting. Smoking status, case urgency status, as well as therapy group were used in the final multivariate regression analysis.
All analyses were conducted with the use of Stata software, version 12 (StataCorp). A two-sided P value of less than 0.05 was considered to indicate statistical significance.
Baseline patient demographics, comorbid conditions, medications and frailty were equal between the two groups (Tables 1 & 2). Immediately prior to surgery, baseline oxygen saturation, respiratory rate, and peak flow were equal between the two groups (Table 1). Surgical case characteristics showed no significant difference between groups. Intraoperative ventilation study design variables of intubation oxygen concentration, tidal volume, and peak end expiratory pressure were confirmed to be different between the two groups as designed (Table 2). Respiratory rate, which was not an inherent part of the study design, was also significantly higher in the intervention group (13.1 vs 11.1 breaths/minute). This is likely due to the decreased tidal volume in the intervention group. There were not significant differences noted in the amount of muscle relaxant used. The anesthesia records were analyzed for any difference in the amount and type of inhaled anesthetic used between both groups and none were found. There was also no significant difference in the amount of intraoperative intravenous fluids, urine output, estimate blood loss, or net fluid intake between both groups (Table 2), which can all greatly affect pulmonary outcomes.
|
Control |
Intervention |
p-value |
|
|
Demographics |
|||
|
Age |
70.2 + 11.1 |
65.2+ 14.65 |
0.34 |
|
Male Gender |
84.60% |
88.20% |
1.00 |
|
BMI |
25.7+4.8 |
26.6+6.1 |
1.00 |
|
Heart failure |
7.7% |
11.8% |
1.00 |
|
Diabetes |
30.8% |
17.7% |
0.67 |
|
Hypertension |
84.6% |
52.9% |
0.12 |
|
CVA/TIA |
23.1% |
0.0% |
0.07 |
|
CAD/ MI |
23.1% |
17.7% |
1.00 |
|
DVT |
23.1% |
5.9% |
0.29 |
|
CKD/ ESRD |
7.7% |
5.9% |
1.00 |
|
Cirrhosis |
0.0% |
0.0% |
1.00 |
|
Preoperative Frailty |
1.85±1.14 |
1.41±1.33 |
0.37 |
|
Medications |
|||
|
Aspirin |
84.6% |
76.5% |
0.67 |
|
ACE Inhibitor |
53.9% |
35.3% |
0.31 |
|
Beta Blocker |
23.1% |
47.1% |
0.26 |
|
Statin |
84.6% |
64.7% |
0.41 |
|
Pulmonary Characteristics |
|||
|
OSA |
0.0% |
23.5% |
0.09 |
|
Asthma/COPD |
38.5% |
41.2% |
0.88 |
|
Pulmonary HTN |
15.4% |
11.8% |
1.00 |
|
Current Smoker |
38.6% |
47.10% |
1.00 |
|
Past Smoker |
46.1% |
64.7% |
0.31 |
|
Home Oxygen |
7.7% |
5.9% |
1.00 |
|
Inhaler |
30.8% |
41.2% |
0.71 |
|
Base SpO2 |
96.3+2.09 |
96.8+2.7 |
0.31 |
|
Base Peak Flow |
355+161 |
355+194 |
0.84 |
|
Base RR |
17.5+1.2 |
16.2+2.5 |
0.07 |
Table 1 Patient Characteristics
*Abbreviations above for body mass index, prior stroke or transient ischemic attack, coronary artery disease or myocardial infarction, deep venous thrombosis, chronic kidney disease or end stage renal disease, and obstructive sleep apnea. Positive presence of pulmonary hypertension, asthma/COPD, inhaler usage recorded. Baseline oxygen saturation, peak flow and respiratory rate recorded
|
Control |
Intervention |
p-value |
|
|
Open |
69.2% |
82.4% |
0.67 |
|
Abdominal |
46.2% |
35.3% |
0.71 |
|
Elective |
61.5% |
70.6% |
0.70 |
|
Booked Case Length (minutes) |
266+81 |
355+194 |
0.45 |
|
Actual Case Length (minutes) |
201+93 |
216 + 129 |
0.97 |
|
Intubation FiO2 |
80.5+11.7% |
48.9+25.5% |
<0.01* |
|
Set Vt (ml) |
560+85 |
413+50 |
<0.01* |
|
Set RR |
11.1+1.7 |
13.1+1.8 |
0.01 |
|
Set PEEP (cc H20) |
4.8+0.6 |
11.0+ 1.9 |
<0.01* |
|
Muscle Relaxant |
92% |
94% |
0.96 |
|
Average Inhaled Anesthetic |
2.05±1.38% |
2.50±1.55% |
0.42 |
|
Desflurane |
23% |
41% |
0.44 |
|
Sevoflurane |
77% |
59% |
0.44 |
|
Fluids (ml) |
2882±1952 |
2861±2839 |
0.8 |
|
Urine Output (ml) |
395±273 |
334±249 |
0.51 |
|
EBL (ml) |
773±1007 |
560 ±961 |
0.48 |
|
Net Fluids (ml) |
1714±1417 |
1966±2138 |
0.37 |
Table 2 Operative Group Differences
*Intubation FiO2 is the inspired oxygen concentration at time of intubation. Set RR is respiratory rate, Set PEEP is the optimized peak expiratory flow calculated from lowest inflection point. Almost all patients were given muscle relaxants. EBL is an abbreviation for estimated blood loss
There was a significant increase in postoperative atelectasis in the control group compared to the intervention group. There was no difference in measured peak flow between both groups, both on post-operative day 1 and 2, as well as when the mean change from their baseline was compared. There was no difference in postoperative oxygen saturation from baseline or on post-operative day 1. However, on post-operative day 2, the intervention group had on average a higher oxygen saturation at 97.5 compared to 93.4 (p= 0.02) (Table 3). The control group had significantly greater length of stay compared to the intervention group (6.9±5.5 vs 3.3±1.8, p < 0.016). No significant difference was found between groups regarding postoperative complications of re-intubation, pneumonia, sepsis, mortality, unplanned readmission, return to the operating room, or total number of complications. ICU length of stay also differed with an average 2.92 days for control group and 1.6 days for intervention group, this approached significance with a p value of 0.09 (Table 4).
|
Control |
Intervention |
p-value |
|
|
Atelectasis (mean change in RAS score from baseline) |
1.4 |
0.2 |
0.02* |
|
Peak Flow POD1* (mean change in ml from baseline) |
-130 |
-131 |
0.48 |
|
Peak Flow POD2* (mean change in ml from baseline) |
-172.5 |
-123.6 |
0.58 |
|
SpO2 POD1* (mean change from baseline) |
-1.3% |
-2% |
0.78 |
|
SpO2 POD2* (mean change from baseline) |
-0.63% |
-2.8% |
0.14 |
|
POD1 Peak Flow (ml) |
264+ 116 |
285+105 |
0.97 |
|
POD2 Peak Flow (ml) |
250+141 |
285+106 |
0.80 |
|
POD1 SpO2 |
94.2+ 2.9% |
96.1+2.8% |
0.16 |
|
POD2 SpO2 |
93.4+ 2.2% |
97.5+2.7% |
0.02* |
Table 3 Respiratory Outcomes
*Atelectasis measured with a chest x-ray radiologic atelectasis scoring system with preoperative and postoperative Xrays. POD abbreviation for postoperative day. SpO2 abbreviation for oxygen saturation
|
Control |
Intervention |
p-value |
|
|
Re-intubation |
7.7% |
0% |
0.43 |
|
Pneumonia |
7.7% |
0% |
0.43 |
|
Sepsis |
0% |
0% |
1.00 |
|
LOS |
6.92±5.48 |
3.29±1.76 |
0.016* |
|
ICU LOS |
2.92±2.13 |
1.61±1.38 |
0.09 |
|
Mortality |
0% |
5.9% |
1.00 |
|
Unplanned readmission or return to OR |
15.4% |
5.9% |
0.56 |
|
Total complications |
30.8% |
11.8% |
0.36 |
Table 4 Clinical Outcomes
*Total complications: Pneumonia, sepsis, reintubation, mortality, readmission or return to OR. LOS is abbreviation for length of stay
|
Odds Ratio |
p value |
|
|
Therapy |
-3.38 ± 1.48 |
0.03* |
|
Frailty Index |
0.47 ± 0.60 |
1.71 |
|
Case Urgency |
0.52 ± 1.56 |
0.74 |
Table 5 Multivariate Regression of Length of Stay
*Therapy group showing significant reduction in length of stay
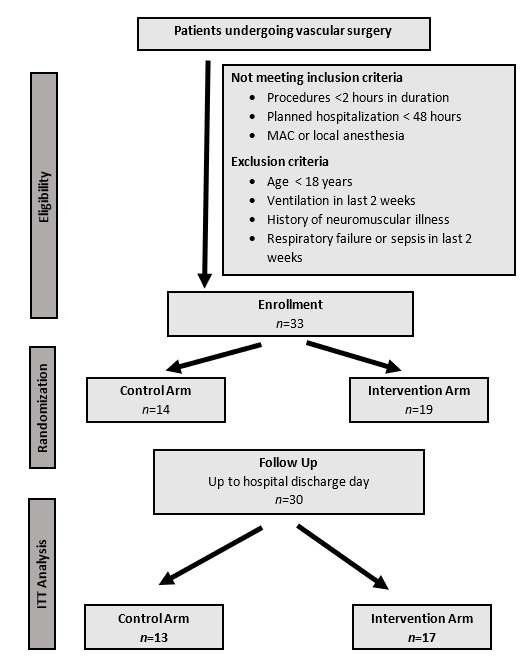
Figure 1 Flow chart representation of study participants enrolled into control and intervention arms that met follow-up and exclusion criteria.
Multivariate regression of factors predicting length of stay included frailty index, case urgency as well as presence in the therapy group. When controlling for smoking status and case urgency, presence in the therapy group was found to significantly affect length of stay in the hospital.
Our hypothesis was that adoption of intra-operative lung-protective ventilation strategies, similar to current ICU ventilation management, would decrease post-operative pulmonary complication rates in patients undergoing major vascular surgery procedures. Due the paucity of literature regarding this clinical question, we designed a trial designed to determine safety and feasibility of the use of protective lung ventilation strategies intra-operatively. We based the study’s therapy choices on the most promising factors from our experience and literature review. From the PROVE network studies, it was clear that lower tidal volume or variable PEEP intraoperatively did not alone seem affect postoperative pulmonary complications.3,4,12,13 The IMPROVE trial involved 400 patients undergoing laparoscopic and open surgery that showed lung protective tidal volumes of 6-8ml/kg of ideal body weight, PEEP of 6-8 and recruitment maneuvers was associated with a 17% reduction in pulmonary and extrapulmonary complications, as well as a 69% reduction in reintubation and postoperative CPAP for postoperative respiratory failure.14 Studies have shown that too high of PEEP can have deleterious effects such as the PROVHILO trial showing that excessive levels of PEEP can compromise hemodynamic parameters, due to effects on venous filling.12 Determination and use of an “optimal” level of positive end expiratory pressure (PEEP) has been used for treatment acute respiratory distress syndrome (ARDS) for several years.15 Our goal was to find a pressure at which lung alveoli are maximally recruited, yet not overdistended. To-date, optimal PEEP has been utilized as a treatment for hypoxia, but not as an intra-operative strategy in non-hypoxic patients. However, it was our hypothesis that optimal alveolar recruitment will benefit intraoperative ventilation management and techniques such as the one we chose for this study were feasible during operative cases of adequate length. Our choice to include a low fraction of inspired oxygen came in part based on ICU literature that has long linked oxygen toxicity to mortality,19 and more recently from studies that have shown intraoperative high FiO2 is associated in a dose-dependent manner with major respiratory complications and with 30-day mortality.20,21
Additionally, the intervention group also had decreased postoperative atelectasis compare to their baselines. Induction of general anesthesia usually reduces lung volume and results in the formation of atelectasis, which may increase further during surgery.22 Atelectasis results in reduced gas exchange and increased inflammation and can persist into the post-operative period. The presence of lung collapse can favor development of pneumonia and bacterial translocation.23,24 In the intraoperative period, atelectasis may promote ventilator induced lung injury due to cyclic collapse and re-opening of lung tissue.22 In this study, the combination of low tidal volume, optimized positive end expiratory pressure (PEEP) and low intraoperative FiO2 led to a decreased amount of postoperative atelectasis according to a double blinded radiologist interpreting the chest radiographs. This may be the cause of the finding of significantly increase oxygen saturation on postoperative day two in the intervention group compared to the control group. Although this did not translate into a perceptible difference in postoperative pulmonary complications such as pneumonia or re-intubation, it corroborates our hypothesis that the combination of factors in the intervention group leads to less atelectasis and therefore less postoperative pulmonary complications.
The findings showed a significant difference in hospital length of stay between control and therapy groups. This is similar to the IMPROVE trial findings of 2013 that also found a significant decrease in hospital stay.14 In our study’s case this is a remarkable finding given our smaller sample size. To evaluate for the absence of Type 1 bias, a multivariate regression was performed, controlling for smoking and case urgency. Multivariate regression showed the intervention group had significantly shorter length of hospital stay (-2.90 days ± 0.18, p = 0.007). Additionally, at a p value of 0.09, ICU length of stay also began to approach significance. Mechanical ventilation itself can induce an inflammatory response and can synergize with the response induced by major surgery at both local and systemic levels. This amplification of the inflammation cascade is thought to contribute to the subsequent development of lung injury and systemic organ failure.23 A difference in systemic inflammation may have contributed to the difference in length of stay between both groups.
Based on the findings of this study, we can introduce new strategies of ventilation protection, including optimal PEEP, recruitment maneuvers, routine use of neuromuscular blockade, in patient at high risk for pulmonary complication in the vascular surgery cohort.
There are several limitations to this study. Due to our low sample size, there is risk for both type 1 and type 2 errors. Although our findings reach significance in a patient sample size of 30, it is likely inadequately powered to change clinical practice. A multivariate regression was run in order to control for other confounding factors and to reduce type 1 bias, only three factors were able to be included due to the patient sample size. As a result, there may be other factors potentially affecting the outcome that were unable to accounted for in the multivariate regression. We found no difference between the two groups for our composite primary outcomes, likely the most relevant clinical outcomes. No significant difference was found between groups regarding postoperative complications of re-intubation, pneumonia, sepsis, mortality, unplanned readmission, return to the operating room, or total number of complications. However, our study was underpowered to identify these differences. For example, there was difference of total complications of 30.8 % in the control group versus 11.8% in the intervention group, but did not achieve significance (P = 0.36).
Our study lays the groundwork for future RCTs in the field of lung protective ventilation, particularly in patients undergoing vascular surgery. Although several international RCTs have studied various individual aspects of protective ventilation in abdominal surgery, few showed difference in postoperative complications. Our study used a novel combination of lung protective techniques based on the most promising aspects of lung protective ventilation literature. In addition, we conducted our study in the vascular surgery population, which is particularly vulnerable to postoperative pulmonary complications. It may be both our combination of interventions and our choice of patient population the led to these findings of significant benefit in our intervention arm. This trial may be underpowered to change clinical practice, it has provided a basis and model for other RCTs. This line of research to promote individualized ventilation care could have significant on vascular surgery outcomes, which is a major interest for hospitals, anesthesiologists and surgeons.
None.
None.

©2021 Saha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.