Journal of
eISSN: 2373-6437


Clinical Paper Volume 13 Issue 1
1Clinical Research Fellow in Critical Care Medicine, Philippine Heart Center, Philippine
2Chair, Division of Critical Care Medicine, Philippine Heart Center, Philippine
Correspondence: Bernard Benjamin P. Albano, Clinical Research Fellow in Critical Care Medicine, Philippine Heart Center, East Avenue, Quezon City 1100, Philippine, Tel 09175665103
Received: January 17, 2021 | Published: February 12, 2021
Citation: Albano BBP, Habana LMI. Prediction of fluid responsiveness in the immediate post-operative period of cardiac surgery. J Anesth Crit Care Open Access. 2021;13(1):35‒45. DOI: 10.15406/jaccoa.2021.13.00467
Background: Prediction of fluid responsiveness can identify patients who will benefit from fluid loading in the immediate postoperative period of cardiac surgery. Several hemodynamic parameters may help identify those who will benefit from hydration. This study aimed to identify and compare the parameters that predict fluid responsiveness in post-cardiac surgery patients.
Methodology: This prospective cohort study included 101 post-cardiac surgery patients. Hemodynamic parameters were recorded at baseline and after an 8 mL/kg IV fluid challenge. Fluid responders are those with an increase in stroke volume of ≥15%. Multivariate analysis was used to identify independent predictors of fluid responder status. Sensitivity and specificity analyses were done to determine the predictive accuracy of each parameter.
Results: The rate of fluid responsiveness was 54.5%. Independent predictors were: central venous pressure (CVP) ≤6 mmHg (p=0.001), pulmonary artery occlusion pressure (PAOP) ≤12 mmHg (p=0.016), PAOP increase by ≥7 mmHg (p=0.002), pulse pressure variability (PPV) >12% (p<0.001), PPV decrease by >5% (p=0.049) and weight (p=0.04). PPV was the most sensitive (92%) and specific (74%); while PAOP was the least sensitive (70%) and CVP the least specific (51%). PPV had the highest ability to discriminate fluid responders (AUC 0.83) compared to PAOP (AUC 0.21) and CVP (AUC 0.40) (p<0.0001).
Conclusion: PPV (a dynamic index) is superior to CVP and PAOP (static indices) in discriminating fluid responders in adult patients who underwent cardiac surgery. PPV is the favored tool to guide initiation of fluid therapy in this clinical setting.
Keywords: pulse pressure variability, central venous pressure, pulmonary artery occlusion pressure, fluid responsiveness
Fluid replacement remains the single most important therapeutic intervention in unstable, critically-ill patients including those in the immediate post-operative period of cardiac surgery.1 It increases intravascular volume resulting to the improvement of cardiac output and tissue perfusion which translates to a more optimum hemodynamics of the patient. However, giving fluids does not always increase cardiac output as one expects.2 In fact, only fifty percent (~50%) of these patients will probably benefit from fluid loading3 while the rest will suffer from the danger of over hydration: acute heart failure, interstitial edema, increased hospital or ICU stay, and even death.3,4 Therefore, predicting fluid responsiveness (also called preload responsiveness) is an important concept that aims to identify those who are truly volume-deficient (also called fluid-responders). The goal is to identify patients who are at the steep portion of the Frank-Starling curve (area of fluid responsiveness, see Figure 1) so they may be given adequate hydration; while the non-responders (those at the plateau portion of the curve) should be given a more stringent fluid management in order to avoid the harmful effects of excess fluids.1,4,5
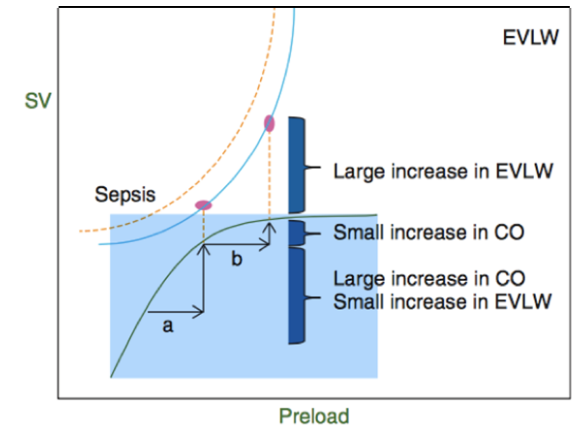
Figure 1 Combined Frank-Starling and Marik-Phillips curves demonstrating the physiology of fluid responsiveness and how over-hydration produces detrimental effects. The Frank-Starling curve (curve within the blue region) shows a steep portion (a) and a plateau portion (b). The steep portion shows a region of fluid responsiveness. As preload increases, there is also a large increase in stroke volume [SV] (or cardiac output [CO]). The plateau portion represents a region of less fluid responsiveness. In this region, further increases in preload no longer produce a large increase in SV.5 The Marik-Phillips curve (curve above) demonstrates that as patients become less fluid responsive, extravascular lung water (EVLW) and tissue edema can markedly increase because the increased cardiac filling pressures causes transmission of hydrostatic pressures.15 This process is emphasized in patients with endothelial damage such as those with sepsis, ARDS and burns.16,17
There are several parameters that predict fluid responsiveness, which can be divided into static and dynamic parameters. Static measurements of preload include central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP, previously known as pulmonary capillary wedge pressure or PCWP). They are traditional filling pressures first described by Mangovern in 19596 and popularized by Weil and Henning in 1979, they became the standard tool to guide fluid therapy since then, and are still used today despite their questionable value in this area.7-11 Both are good estimates of preload but their capacity to predict fluid responsiveness may be inaccurate because they are only surrogate measures.4,9-11 This is especially true in the mechanically-ventilated post-cardiac surgery patients due to the wide variations of intra-thoracic pressures and changes in the geometry and structure of the heart as a result of the fluid changes, the cardiopulmonary bypass and the surgery itself. Dynamic measures such as pulse pressure variation (PPV) and stroke volume variation (SVV) have shown promise in this area and has been a subject of interest in the fluid response of critically-ill and post cardiac surgical patients.7 Mechanical ventilation produces cyclic variations in cardiac preload that are manifest as changes in stroke volume and arterial pulse pressure. This variation in arterial pulse pressure has been shown to be valuable in discriminating patient responsiveness to fluid loading.8 The higher the variability equates to a more volume deficient state.
This study aimed to identify and compare the various clinical and hemodynamic predictors of fluid responsiveness during the immediate postoperative period of cardiac surgery, in order to identify which tool is the most accurate to guide fluid therapy in this clinical setting.
Ethical considerations: This study was conducted in compliance with the ethical principles set forth in the Declaration of Helsinki. Prior to the study invitation, the protocol was reviewed and approved by the Philippine Heart Center Institutional Ethics Review Board (PHC IERB). Prior to the conduct of the study, an informed consent was taken from all patients. The risk to the subject’s privacy was minimal and no sensitive information were obtained. The investigator ensured that the subject’s anonymity was maintained.
Study design: Figure 2 demonstrates the study design. This was a prospective cohort study which included 101 adult patients who underwent cardiac surgery. The follow-up involved a 30-minute postoperative period which covered the initial/baseline clinical and hemodynamic measurement, fluid resuscitation and repeat hemodynamic assessment.
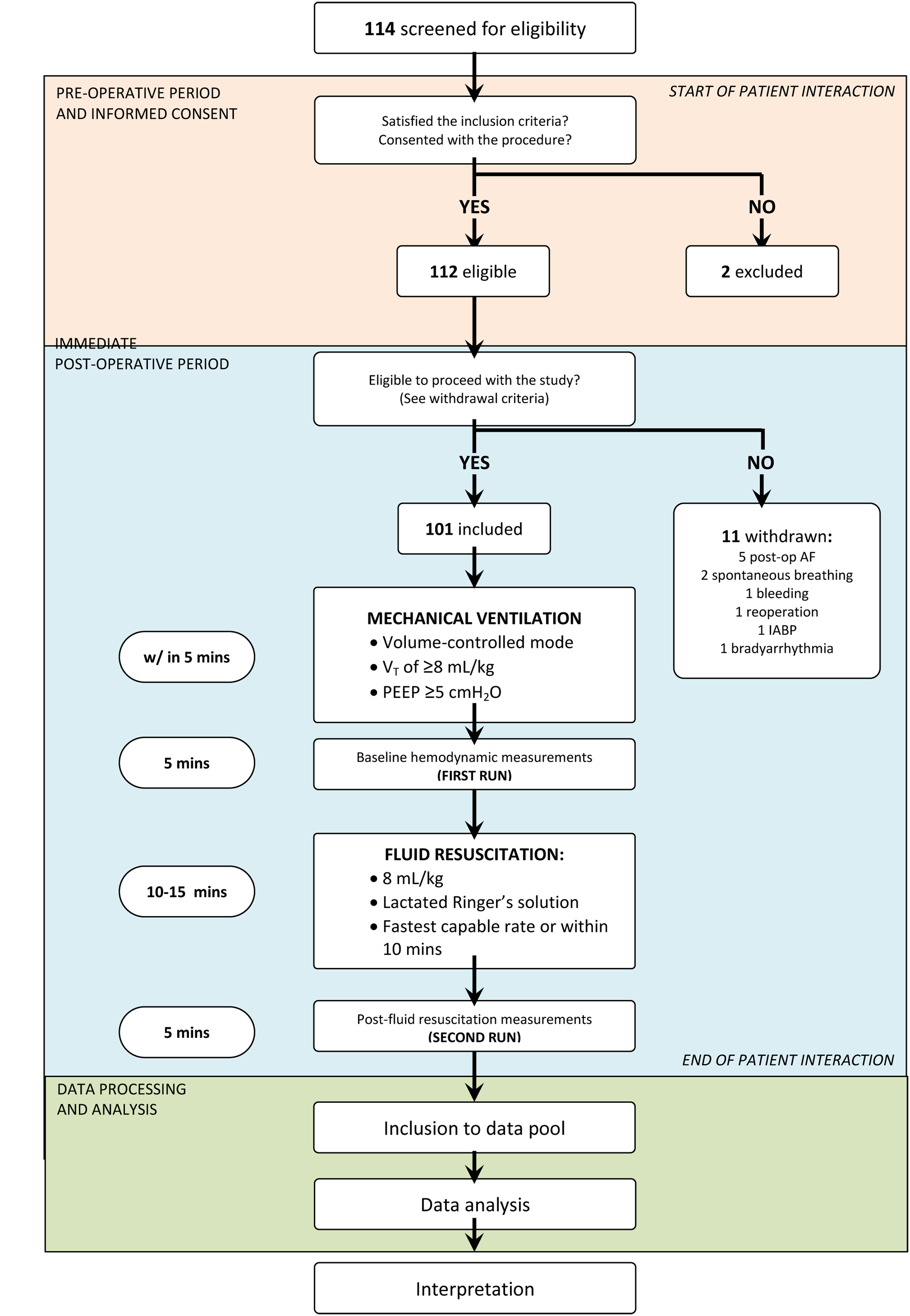
Figure 2 Study design and enrollment. This panel shows the step-by-step process from eligibility screening to data collection and analysis. The expected time of accomplishment for each step during the post-operative period is also indicated.
Setting and time period: The study consceptualization started on June 2017 and was approved by the technical committee and ethics board on August 2017. The data collection started on September up to December 2017.
Subject selection: Figure 2 demonstrates the screening for eligibility and enrollment process.
Inclusion criteria: Patients included were ≥19 years of age who underwent cardiac surgery (coronary artery bypass graft, valvular surgery, ASD and VSD patch closures; and vascular surgery [aortic repair or replacement]) whose preoperative risk was low; and completeley sedated and hooked to mechanical ventilation after surgery. The patients were attached to cardiac monitor (Nihon Kohden Lifesope©) capabale to measure the following indices: PPV, CVP, PAOP and cardiac output or cardiac index using standard thermodilution method (Td).
Exclusion criteria: Patients were excluded if factors that predispose to inaccurate measurement of cardiac output using the Td technique were present, including low cardiac output, low ejection fractions (EF ≤40%) on preoperative assessment, if the patient had spontaneous breathing activity, presence of sustained arrythmias, open chest, and presence of residual severe tricuspid or any valvular regurgitations.
Withdrawal criteria: After surgery, patients were withdrawn from the study if they had the following conditions: (1) on mechanical circulatory support (intraaortic balloon counterpulsation [IABP] and/or extracorporeal membrane oxygenation [ECMO]); (2) unstable post-operative course (frequent fatal arrhythmias, intractable hypotension requiring high dose vasopressors and inotropes); (3) Post-operative complications such as uncontrolled bleeding, severe neurologic impairment, or accidental mechanical complications; and (4) prolonged operative time.
Study maneuver: Figure 2 summarizes how this study was conducted from recruitment to analysis.
Mechanical Ventilation: Patients included were immediately hooked to mechanical ventilation using a volume-controlled mode with a tidal volume of ≥ 8 mL/kg and a positive end-expiratory pressure of ≥ 5 cmH2O (12). Those with spontaneous breathing activity were excluded through visual inspection of the airway pressure curve on the ventilator and of the capnographic signal on the bedside monitor.
Baseline characteristics: Once on mechanical ventilation, demographic and anthropometric data, admission diagnosis and surgical diagnoses were recorded.
Hemodynamic measurements: Baseline hemodynamic parameters were recorded as well: hemodynamic drug usage, pulse pressure variation (PPV), central venous pressure (CVP), pulmonary artery occlusion pressure (PAOP), cardiac output (CO), cardiac index (CI), stroke volume (SV), systemic vascular resistance (SVR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and cardiac rate (CR). The measurement of cardiac output were done twice: before and immediately after fluid challenge. Comparison of hemodynamic variables before fluid resuscitation in responder and non-responder patients was assessed.
Thermodilution (Td) technique: The measurement of cardiac output was carried out using standard thermodilution technique.5 Td was the chosen method of hemodynamic assessment because it remains as the gold standard procedure for measuring CO.13 Right-sided cardiac catheterization was achieved using a triple-lumen balloon-tipped pulmonary artery catheter (PAC) (American-Edwards Laboratories) inserted via the internal jugular vein. Catheter tip was positioned either in the main pulmonary artery or in the proximal right/left main pulmonary artery. Catheter tip placement was verified by pressure tracing or by x-ray visualization. Re-positioning was done by adjusting the catheter so that it required full inflation of the balloon in order to obtain a pulmonary artery occlusion pressure. Multiple syringes containing 10 mL of cold normal saline were used as injectate, connected to the barrel of the PAC system. The rate of injection was less than four seconds during end-expiration. When the first three injections produced stroke volumes (SV) that are within 10% variation, the average of the three determinations was used as the cardiac output (CO) measurement. If the variation was more than 10%, additional two injectates (also containing 10 mL of normal saline) were done, and the average of the three middle determinations (highest and lowest values disregarded) was used as CO measurements.
Fluid challenge: Intravascular volume expansion was done after measurement of baseline hemodynamic parameters using 8 mL/kg of lactated Ringer’s solution delivered intravenously via the fastest capable rate.5
Definition of outcomes: The outcome variable was the patients’ hemodynamic response to volume expansion defined by an increase in SV or CI of 15% or more after the fluid challenge.9 If this was satisfied, the patient was labeled “responder” or “fluid-responsive”. Fluid responsiveness was manually recorded as a binary variable (“yes” or “no”). Independent variables of this study include measurement of hemodynamic parameters described above: SBP, DBP, MAP, CR, CO, CI, SV, SVR PAOP, CVP and PPV. Confounding variables may be present in the subjects that might contribute to misinterpretation of tests such as early awakening (which might affect the cyclic variation induced by mechanical ventilation), presence of paroxysmal atrial fibrillation not seen during the determination of the hemodynamic parameter and underestimation of regurgitant lesions after surgery, which might affect the estimation of CO. The hemodynamic parameters that predict fluid responsiveness were identified and they predictive accuraries were compared in this study.
Sample size calculation: A total of 81 subjects were required for this study based on a level of significance of 5%,14 an area under the curve of 0.64 and a 95% confidence interval of 0.53 to 0.74 as noted from the reference article by Cherpanath et al.1 The total subjects enrolled in this study was 101.
Statistical analysis. Descriptive statistics was used to summarize the demographic and clinical characteristics of the patients. Frequency and proportion were used for categorical variables and mean+SD for normally distributed continuous variables. Independent T-test and Fisher’s exact test were used to determine the difference of mean and frequency, respectively, between fluid responder status and before & after fluid challenge. Odds ratio and corresponding 95% confidence intervals from binary logistic regression was computed to determine significant predictors for fluid responsiveness. Sensitivity, Specificity, NPV, PPV, and likelihood ratios were determined for each hemodynamic parameter. The accuracies of the different dynamic and static parameters were measured and the area under the receiver operating characteristic curves determined and compared. Missing variables was neither replaced nor estimated. Null hypotheses were rejected at 0.05α-level of significance. STATA 13.1 was used for data analysis.
Patients: A total of 114 patients were screened for eligibility. Two patients revoked their consent even before surgery and were excluded from the study and 112 were eligible and were followed up after surgery. A total of 11 patients were withrawn because of the presence of factors that could impair the interpretation of data. Of these, 5 patients had post-operative atrial fibrillation; 2 had spontaneous breathing activity; 1 had severe bleeding; 1 needed reoperation; another had severe bradyarrhythmia warranting temporary pacing; and another needed mechanical circulatory support (intra-aortic balloon counterpulsation) (Figure 2).
Fluid responder status: Of the 101 patients, 55 satisfied the definition of fluid responsiveness. The rate of fluid responsiveness of the population is 54.5%.
Baseline characteristics: Table 1 demonstrates the clinical and demographic characteristics of patients at baseline.
|
Parameter (N=101) |
R |
NR |
p value |
|
Frequency (%) or Mean ± SD |
|||
|
Demographics |
|
|
|
|
Age (years) |
57.12 ± 10.29 |
54.23 ± 13.89 |
0.43 |
|
Weight (kg) |
68.33 ± 11.42 |
62.32 ± 12.59 |
0.01* |
|
Height (cm) |
162.02 ± 9.45 |
157.60 ± 15.96 |
0.09 |
|
Body surface area (m2) |
1.75 ± 0.18 |
1.64 ± 0.22 |
0.008* |
|
Temperature (‘C) |
36.28 ± 1.25 |
36.44 ± 0.95 |
0.51 |
|
Gender (Male) |
45 (78.26) |
30 (68.18) |
0.22 |
|
Risk Factors |
|
|
|
|
Hypertension |
44 (77.19) |
33 (75) |
0.80 |
|
Diabetes mellitus |
23 (40.35) |
19 (43.18) |
0.77 |
|
Smoker |
17 (29.82) |
18 (40.81) |
0.25 |
|
Dyslipidemia |
19 (33.33) |
24 (54.55) |
0.07 |
|
Family history of IHD |
1 (1.75) |
1 (2.27) |
0.64 |
|
Diagnosis |
|
|
|
|
Stable CAD |
45 (78.95) |
34 (77.27) |
0.91 |
|
Valvular heart disease |
8 (14.04) |
5 (11.36) |
|
|
Congenital heart disease |
3 (5.26) |
4 (9.09) |
|
|
Vascular/Aortic disease |
1 (1.75) |
1 (2.27) |
|
|
Echo parameters (pre-op) |
|
|
|
|
LVEF (%, Simpson’s) |
59.34 ± 12.86 |
59.73 ± 10.18 |
0.87 |
|
LVEDD |
5.96 ± 6.97 |
4.77 ± 0.93 |
0.28 |
|
LAVI (mL/BSA) |
35.19 ± 40.26 |
32.38 ± 23.48 |
0.73 |
|
RVFAC (%) |
50.67 ± 6.26 |
53.43 ± 9.24 |
0.13 |
|
Vasoactive drug use |
|
|
|
|
Norepinephrine |
14 (24.56) |
6 (13.64) |
0.17 |
|
Epinephrine |
0 (0) |
3 (6.82) |
0.08 |
|
Dobutamine |
40 (70.18) |
21 (47.73) |
0.02* |
|
Milrinone |
0 (0) |
2 (4.55) |
0.19 |
|
Nitroglycerine (NTG) |
36 (63.16) |
27 (61.36) |
0.51 |
|
Nicardipine |
13 (22.81) |
13 (29.55) |
0.44 |
|
Type of surgery |
|
|
|
|
CABG (On-pump) |
41 (71.93) |
32 (72.73) |
0.23 |
|
CABG (Off-pump) |
5 (8.77) |
0 (0) |
|
|
Valvular surgery |
8 (14.04) |
7 (15.91) |
|
|
Congenital surgery |
2 (3.51) |
4 (9.09) |
|
|
Vascular/aortic surgery |
1 (1.75) |
1 (2.27) |
|
|
Duration of surgery |
|
|
|
|
Total OR time (min) |
337.57 ± 70.41 |
325 ± 105.37 |
0.57 |
|
CPB time (min) |
142.02 ± 49.17 |
133.63 ± 47.83 |
0.41 |
|
AXC time (min) |
113.18 ± 34.96 |
105.88 ± 39.66 |
0.35 |
|
Volume resuscitation given (mL) |
546.67 ± 91.39 |
498.53 ± 100.73 |
0.01 |
Table 1 Baseline characteristics of the study population determined before fluid resuscitation comparing the responders (R) and non-responders (NR)
* statistically significant
IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic dimension; LAVI, left atrial volume index; RVFAC, right ventricular fractional area change; PASP, pulmonary artery systolic pressure; CABG, coronary artery bypass graft; CBP, cardiopulmonary bypass; AXC, aortic cross clamp
Demographics: There was no significant difference between the two groups in terms of age, height and gender. The mean weight in the responder group was higher (68.33 ± 11.42 Kg) compared to the non-responders (62.32 ± 12.59 Kg), and this difference was statistically significant (p=0.01). The body surface area (BSA) also was higher for responders (1.75 ± 0.18 Kg/m2) compared to non-responders (1.64 ± 0.22 Kg/m2) and the difference was statistically significant (p=0.008).
Risk factors: The most common risk factor was hypertension which had a prevalence of 77% in the responders and 75% in the non-responders. This was followed by diabetes mellitus with a prevalence of 40% in the responders and 43% in the non-responders. Fewer patients (30%) had a history of smoking in the responder groups compared to nonresponders (41%) but this was not statistically significant. Other risk factors such as dyslipidemia and family history of IHD were not significantly different between the two groups.
Diagnosis: Most common diagnosis was coronary artery disease (79% in responders and 77% in non-responders), this was followed by valvular heart disease (14% in responders and 11% in non-responders). There was no significant difference between the two groups in terms of diagnoses.
Echocardiographic parameters: There were no significant difference in the echocardiographic parameters between the two groups as shown in Table 1.
Vasoactive drug use: The most commonly used vasoactive drug at the surgical ICU was dobutamine. More patients in the responder group (70%) were given dobutamine compared to the non-responders (48%), and this difference was statistically significant (p=0.02). The next most commonly used drug was nitroglycerine followed by nicardepine and norepinephrine. Aside from dobutamine, there was no significant difference between the two groups in terms of vasoactive drug use.
Type of surgery: The most common procedure done was CABG (on-pump). Seventy-two percent of the responder group while 73% of the non-responder group underwent this procedure. The next most common procedure is valvular surgery followed by congenital surgery and aortic procedures. Nevertheless, their differences between the two groups were not statistically significant.
Duration of surgery: The responder group had a slightly longer cardiopulmonary bypass time (142 minutes) and cross clamp time (113 minutes) compared to non-responders (144 minutes and 106 minutes, respectively). Nevertheless, their differences were not statistically significant. Also, the total operative time was not significantly different between the two groups.
Nevertheless, their differences were not statistically significant. Also, the total operative time was not significantly.
Fluid challenge volume: The computed volume to be given to the patients during the fluid challenge was 8 ml/Kg. There was a slightly higher mean volumes given to responders (547 mL) compared to non-responders (500 mL) and this difference was statistically significant.
Baseline hemodynamic parameters: Table 2 demonstrates the baseline hemodynamic and tissue perfusion parameters of the population.
|
Parameter (N=101) |
R |
NR |
p-value |
|
Frequency (%) or Mean ± SD |
|||
|
Vital parameters |
|||
|
Cardiac rate (bpm) |
88.18 ± 17.83 |
84.34 ± 19.13 |
0.30 |
|
SBP (mmHg) |
136.82 ± 29.95 |
134.84 ± 25.18 |
0.73 |
|
DBP (mmHg) |
73.84 ± 13.68 |
68.45 ± 14.58 |
0.06 |
|
MAP (mmHg) |
94.80 ± 17.64 |
90.48 ± 16.15 |
0.21 |
|
Cardiac output parameters (Thermodilution method) |
|||
|
CO (L/min) |
3.83 ± 1.19 |
3.96 ± 1.03 |
0.57 |
|
CI (L/min/m2) |
2.21 ± 0.73 |
2.43 ± 0.61 |
0.13 |
|
SV (mL) |
41.72 ± 14.73 |
46.39 ± 16.33 |
0.16 |
|
SVR (Dynes/sec/cm-5) |
1861.68 ± 635.84 |
1746.07 ± 607.62 |
0.36 |
|
Baseline filling pressure parameters |
|||
|
CVP (mmHg) |
9.56 ± 5.03 |
11.02 ± 3.99 |
0.12 |
|
PAOP (mmHg) |
8.81 ± 4.22 |
13.16 ± 4.13 |
<0.001* |
|
PPV (%) |
16.28 ± 6.46 |
8.95 ± 3.78 |
<0.001* |
|
Tissue perfusion parameters |
|||
|
SVO2 (%) |
70.56 ± 8.93 |
72.03 ± 10.64 |
0.45 |
|
SVO2 < 65% |
17 (29.82) |
11 (25.0) |
0.59 |
|
PCO2 gap (mmHg) |
6.36 ± 3.30 |
6.52 ± 4.44 |
0.84 |
|
PCO2 gap > 5 mmHg |
36 (64.29) |
23 (52.27) |
0.23 |
Table 2 Baseline hemodynamic and tissue perfusion parameters of the study population comparing the responders (R) and non-responders (NR) |
Vital paramaters: There were no significant difference in the baseline vital paramaters of the population including cardiac rate, systolic and diastolic BP and mean arterial pressure.
Cardiac output measurements: The baseline cardiac output measurements (cardiac output/index, stroke volume and systemic vascular resistance) were also not significantly different between the two groups.
Baseline cardiac filling pressures: The mean central venous pressure (CVP) in the responder group was lower (9.56 ± 5.03 mmHg) compared to the non-responder (11.02 ± 3.99 mmHg). However this difference was not statistically sigificant (p=012). The pulmonary artery occlusion pressure (PAOP) was lower in the responder group (8.81 ± 4.22 mmHg) compared to non-responders (13.16 ± 4.13 mmHg) (p<0.001). The pulse pressure variability (PPV), which is a dynamic filling pressure parameter was higher in the responder group (16.28 ± 6.46%) compared no non-responders (8.95 ± 3.78%) (p<0.001).
Tissue perfusion paramaters: These parameters include mixed venous O2 saturation (SVO2) (normally >65%) and PCO2 gap (normally <5 mmHg). A low SVO2 and a high PCO2 gap variably predict mortality in critically-ill patients. However, both baseline parameters and categorical values were not different between responders and non-responders.
Categorized values of filling pressures: Table 3 demonstrates the categorical values of the 3 filling pressures. In this table, the effect of low baseline value (low CVP, low PAOP), as well as the degree of increase from their baseline values after fluid challenge were evaluated for prediction of fluid responsiveness. In contrast, the PPV was categorized as (high baseline PPV, >12%) and decreased from baseline value after fluid challenge.
|
Parameter (N=101) |
R |
NR |
p-value |
|
Frequency (%) or Mean ± SD |
|||
|
Central venous pressure (CVP) |
|||
|
Baseline CVP ≤ 6 mmHg |
19 (33.33) |
4 (9.1) |
0.003* |
|
CVP increased by 2-5 mmHg |
36 (63.16) |
34 (77.27) |
0.10 |
|
CVP increased by >5 mmHg |
17 (29.82) |
4 (9.09) |
0.009* |
|
Pulmonary artery occlusion pressure (PAOP) |
|||
|
Baseline PAOP ≤12 mmHg |
45 (78.95) |
19 (43.18) |
<0.0001* |
|
PAOP increased by 3-7 mmHg |
31 (54.39) |
40 (90.91) |
<0.001* |
|
PAOP increased by >7 mmHg |
23 (40.35) |
2 (4.55) |
<0.001* |
|
Pulse pressure variability (PPV) |
|||
|
Baseline PPV >12% |
43 (75.44) |
4 (9.09) |
<0.001* |
|
PPV decreased to ≤10% |
39 (68.42) |
8 (18.18) |
<0.001* |
|
PPV decreased by > 5% |
48 (84.21) |
10 (22.73) |
<0.001* |
Table 3 Categorical values of the different hemodynamic parameters comparing the responders (R) and non-responders (NR). The different filling pressures were categorized to determine the effect of low baseline CVP and PAOP (or high baseline PPV) and the degree of change from their baseline values after fluid challenge in predicting fluid responsiveness |
Central venous pressure: The categorical value of low baseline CVP (≤ 6 mmHg) was more common in the responder group (33%) compared to non-responders (9%) (p=0.003). Also the baseline CVP value increase by >5 mmHg was found to be more common (30%) in the responders compared to non-responders (9%) (p=0.009). The CVP increase of 2-5 mmHg was not different between the 2 groups.
Pulmonary artery occlusion pressure: The categorical value of low baseline PAOP (≤12 mmHg) was more common in the responder than non-responder groups. The baseline PAOP increase by 3-7 and >7 mmHg were also noted to be more common in the responder group (p<0.001).
Pulse pressure variability: This “dynamic” filling pressure’s relationship with fluid resposiveness is direct (in contrast to CVP and PAOP, which are indirect). The higher the variability equates to a more fluid responsive status. After categorizing the PPV value (high PPV [>12%]) we noted that a high PPV was more common in the responder group (75%) compared to the non-responder group (10%) (p<0.001). Also, the effect of changes in PPV value after fluid challenge appear to predict fluid responsiveness. The PPV decrease to a value lower or equal to 10% happened more commonly in the responder groups (68%) than in non-responders (18%) (p<0.001). The PPV decrease by 5% or more was set arbitrarily to further determine the predictive effect of PPV changes on fluid responsiveness. Similarly, there were more patients (88%) in the responder group who had a decrease of PPV by 5% or more compared to the non-responders (35%), and this difference was statistically signifiant (p<0.001).
Independent predictors of fluid responsiveness: Table 4 demonstrates the univariate and multivariate regression analyses to identify the independent predictors.
|
Parameter |
Univariate |
Multivariate |
||||
|
Crude Odds Ratio |
95% confidence interval |
p value |
Adjusted Odds Ratio |
95% confidence interval |
p value |
|
|
Baseline CVP (mmHg) |
0.93 |
0.85 – 1.02 |
0.12 |
- |
||
|
CVP ≤ 6 mmHg |
5.0 |
1.56 – 16.05 |
0.007* |
52.80 |
5.09 – 547.32 |
0.001** |
|
CVP increase by > 5 mmHg |
4.25 |
1.31 – 13.75 |
0.016* |
- |
||
|
Baseline PAOP (mmHg) |
0.78 |
0.69 – 0.88 |
<0.005* |
- |
||
|
PAOP ≤12 mmHg |
4.93 |
0.40 – 7.1 |
<0.005* |
5.40 |
1.36 – 21.40 |
0.016** |
|
PAOP increase by ≥ 7 mmHg |
14.21 |
3.13 – 64.56 |
0.001* |
32.43 |
3.76 – 280.09 |
0.002** |
|
Baseline PPV (%) |
1.32 |
1.18 – 1.48 |
<0.005* |
- |
||
|
PPV > 12% |
30.71 |
9.33 – 101.13 |
<0.005* |
23.41 |
4.03 – 136.11 |
<0.001** |
|
PPV change to ≤10% |
9.75 |
3.78 – 25.16 |
<0.005* |
|
- |
|
|
PPV decrease by > 5% |
18.13 |
6.65 – 49.39 |
<0.005* |
5.39 |
1.07 – 27.07 |
0.049** |
|
Weight (Kg) |
1.04 |
1.01 – 1.08 |
0.017* |
1.07 |
1.01 – 1.13 |
0.04** |
|
Dobutamine use |
2.58 |
1.14 – 5.85 |
0.024* |
- |
||
Table 4 Univariate and multivariate analysis to identify independent predictors of fluid responsiveness
* statistically significant
predictors** independent predictors of fluid responsivenessCVP, central venous pressure; PAOP, pulmonary artery occlusion pressure; PPV, pulse pressure variabilityShaded parameters are independent predictors of fluid responsiveness after the multivariate regression analysis
Univariate analysis: Those identified to be statistically-significant predictors of responsiveness in the independent T-test were subjected to univariate regression analysis to identify their linear relationship with fluid responsiveness. Using univariate regression, the hemdynamic parameters noted to be significant were: low baseline CVP (≤ 6 mmHg) (p=0.007), CVP increase by 5 mmHg (p=0.016), mean baseline PAOP (p<0.001), low baseline PAOP (≤12 mmHg) (p<0.001), PAOP increase by ≥ 7 mmHg (p=0.001), baseline mean PPV (p<0.001), high baseline PPV (PPV >12%) (p<0.001), and PPV decrease by >5% (p<0.001). Other parameters that were significant were weight (p=0.017) and dobutamine use (p=0.024).
Multivariate analysis: The parameters that were significant in the univariate regression was subjected to multiple regression to identify the relative contribution of each of the predictors to fluid responsiveness, ultimately to identify the independent predictor of fluid responsiveness. After the multivariate analysis, only five (5) hemodynamic parameters were left to be statistically significant: low baseline CVP (CVP ≤ 6 mmHg) (p=0.001), low baseline PAOP (PAOP ≤12 mmHg) (p=0.016), PAOP increase by ≥ 7 mmHg (p=0.002), high baseline PPV (PPV >12%) (p<0.001), and PPV decrease by >5% (p=0.049). Additionally, weight appeared to be also statistically significant (p=0.04). Altogether, these 6 parameters are the independent predictors of fluid responsiveness in this study. Dobutamine use as well as the mean baseline CVP, PAOP, PPV, CVP increase by >5 mmHg and PPV decrease to absolute value ≤10% were not statistically significant and therefore were omitted.
Predictive accuracy: Table 5 demonstrates the sensitivity and specificity analysis of the different hemodynamic parameters (filling pressures) at a selected cut-off values.
|
Parameter |
Sensitivity |
Specificity |
Positive |
Negative Predictive Value |
|
PPV >12% |
92% |
74% |
75% |
91% |
|
PPV decrease by >5% |
83% |
79% |
84% |
77% |
|
PAOP ≤12 mmHg |
70% |
68% |
79% |
57% |
|
PAOP increase by ≥7 mmHg |
91% |
48% |
40% |
95% |
|
CVP ≤6 mmHg |
83% |
51% |
33% |
91% |
Table 5 Sensitivity and specificity of the filling pressure parameters
CVP, central venous pressure; PAOP, pulmonary artery occlusion pressure; PPV, pulse pressure variability
Sensitivity: As shown in the table, a PPV (>12%) has the highest sensitivity (92%) among the indices in predicting fluid responsiveness. PAOP increase by ≥7 mmHg comes next with 91%, then followed by both PPV decrease by >5% (79%) and CVP (≤6 mmHg) awith 83%. PAOP (≤12 mmHg) was the least sensitive (70%).
Specificity: PPV decrease by >5% was the most sepcific (74%) among the predictors. This is followed by PPV > 12% with 74%. CVP was the least specific (51%).
Positive predictive value: PPV decrease by >5% had the highest positive predictive value (84%) followed equally by PAOP ≤12 mmHg and CVP ≤6 mmHg (91%).
Negative predictive value: PAOP increase by ≥7 mmHg had the highest negtaive predictive value (95%), followed by both PPV and CVP with 91% negative predictive value. PAOP ≤12 mmHg had the least with 57%.
Discriminative ability: Table 6 compares the ability of the CVP, PAOP and PPV in discriminating fluid responders from non-responders, presented in terms of area under the ROC curve (AUC). As seen in the table, PPV had the highest AUC (0.829), followed by CVP (0.398) then by PAOP (0.213). Their differences were statistically significant (p<0.0001). Figure 3 graphically represents their AUCs.
|
Baseline Parameter |
AUC |
95% confidence interval |
P value |
|
PPV (%) |
0.829 |
0.743 – 0.915 |
< 0.0001 |
|
PAOP (mmHg) |
0.213 |
0.124 – 0.302 |
|
|
CVP (mmHg) |
0.398 |
0.288 – 0.509 |
Table 6 Receiver operating characteristics (ROC) values comparing the ability of CVP, PAOP, and PPV to discriminate responder (SV increase of 15% or more) and non-responder patients to IV fluid resuscitation
CVP, central venous pressure; PAOP, pulmonary artery occlusion pressure; PPV, pulse pressure variation; AUC, area under the ROC curve
Clinical predictors: Only four clinical parameters showed significance in predicting fluid responsiveness as shown in Table 1: weight, body surface area, dobutamine use and mean volume of fluid given during the fluid challenge. To our knowledge, there are no other studies that identified weight as predictor of fluid responsiveness. The effect of weight was also significant even after the multivariate analysis (Table 4). It showed that for every unit (Kg) increase in weight, the odds of having fluid responsiveness was increased by 1.1%, controlling for the effects of CVP, PAOP and PPV. Body surface area and volume of fluids given were also identified as significant predictors, however, weight is a function of both parameters and therefore were no longer included in the regression analysis. It is possible that the higher weight equates to a higher volume of distribution which in turn translates to a higher capacity to accommodate volume. Dobutamine use was also identified as a predictor, as expected, because it is an inotropic agent and therefore has an effect on the augmentation of stroke volume and cardiac output. In this study, those given with dobutamine were more likely to have a higher SV and CO during the second hemodynamic assessment (second run). Nevertheless, the effect of dobutamine was omitted in the multivariate regression and therefore was not considered an independent predictor.
Hemodynamic predictors: As demonstrated in Table 2, there were only 2 predictors of fluid responsiveness: mean baseline PAOP and PPV. The mean baseline CVP was not statistically significant which is consistent with the findings of several studies and meta-analyses.17 The tissue perfusion parameters (SVO2 and PCO2 gap), which are predictors of mortality in the critically-ill and post-CABG patients were not statistically significant even after categorizing then into low SVO2 (<65%) and high PCO2 gap (PCO2 gap >5). This means that even if they were predictors of tissue perfusion and mortality in a another study,18 their capacity to predict fluid responsiveness is not adequate to generate significance.
Categorical values of hemodynamic parameters: The value of the hemodynamic predictors were categorized in order to determine the effect of low CVP and low PAOP (or high PPV), and the effect of their degree of change from baseline after the fluid challenge (increase in CVP and PAOP; decrease in PPV). Multivariate analysis identified five hemodynamic parameters that independently predict fluid responsiveness: low baseline CVP (CVP ≤ 6 mmHg), low baseline PAOP (PAOP ≤12 mmHg), PAOP increase by ≥ 7 mmHg (p=0.002), high baseline PPV (PPV >12%), and PPV decrease by >5%.
Effect of Low CVP and increase in CVP: A low CVP (≤ 6 mmHg) was found to be an independent predictor of fluid responsiveness in this study. Table 4 shows that the odds of having fluid responsiveness with a CVP ≤ 6 mmHg is 52 times higher that those with CVP > 6 mmHg. This is in contrast to the finding of Marik9,10,16 where he noted that there was no relationship between the CVP (or any change in CVP) and fluid responsiveness in diverse clinical settings.9,10,16 He mentioned that CVP is a good marker of cardiac preload and cardiac function but not a good predictor of fluid responsiveness. In this study, any changes in CVP were also not statistically significant independent predictors. This supports the finding of Marik14 that the “CVP 2-5 rule” popularized by Weil and Henning in 197913 which has been the traditional scheme to guide fluid therapy, is not a good predictor of fluid responsiveness and therefore we should not rely on these changes to guide fluid therapy. In capsule, this study showed that a low CVP may still have a value in fluid responsiveness prediction in post-cardiac surgical patients, but any degree of changes (or increase) in CVP has no role in the prediction.
Effect of low PAOP and increase in PAOP: Table 4 demonstrates that a low PAOP (≤12 mmHg) as well as increase in PAOP of ≥ 7 mmHg were both a statistically significant independent predictor of fluid responsiveness. The findings of this study defies the assumption of Monnet et al.2 that what is true for the CVP is true for all static indicators of cardiac preload including PAOP (CVP=PAOP). In this study, the odds of being fluid responsive with a PAOP ≤12 mmHg is 5 times higher than having a PAOP > 12 mmHg. Similarly the odds of being fluid responsive with a PAOP increase of ≥7 mmHg is 32 times higher than with a change of only <7 mmHg. Therefore, there is still value of low PAOP and an increase in PAOP in predicting fluid responsiveness as found in this study. The “PAOP 3-7 rule” to guide fluid therapy by Weil and Henning14 may still have a value in guiding fluid therapy. Furthermore, the findings of this study with regard to PAOP questions the assumptions of other studies that all static paramaters are the same.2
Effect of high PPV and decrease in PPV: The first method developed to assess dynamic preload responsiveness was stroke volume variability (SVV), it was in 2000 where PPV was shown to predict the response of cardiac output to fluids.19 And to date, it has the most number of accumulated evidence.6,20 The median threshold of the PPV used in several studies was 12% (interquartile range 10–13%).21 This study demonstrated that a high PPV (>12%) and a decrease in PPV by >5% were significant independent predictors of fluid responsiveness. The decrease of PPV to <10% did not show significance and therefore is not a predictor of response. In this study, the odds of having a fluid response with a PPV >12% is 23 times higher than when PPV is ≤12%. The odds having a fluid response with a decrease of PPV by >5% is 5 times higher than when the decrease is <5%. This change (decrease) in PPV to predict fluid response is a novel finding. The change of PPV to ≤10% was not a significant predictor probably because this decrease does not reflect a true degree of change. For example a PPV decrease of 20% to 9% and a decrease of 11% to 9% both belong to this categorical value even if degree of change of the former is certainly larger than the latter. In summary, a high PPV is an independent predictor of fluid response consistent with other studies, while a change in PPV by >5% is also a predictor as it reflects a true degree of change (decrease).
Predictive accuracy: Table 5 shows the sensitivity and specificity analysis. It is evident that PPV had the highest sensitivity (92%) and specificity (74%) among the three preload indices. A meta-analysis of 22 studies with a total of 807 patients reported that the pooled sensitivity of PPV (cut off value of >12%) for predicting fluid responsiveness was 88% and a specificity of 89%.21 The sensitivity value of PPV here in this study was higher compared to the previously described study but the specificity was a little lower. The highest positive predictive value was PAOP while the highest negative predictive value were both PPV and CVP. The least sensitive was PAOP (cut off value of <12 mmHg) while the least specific was CVP (cut off value of <6 mmHg). In this study, PPV had a superior predictive ability compared to static indices (CVP and PAOP) in identifying fluid responders from non-responders. The PPV cut off of >12% may be used therefore as a cut-off value to predict fluid responsiveness in patients who underwent cardiac surgery.
Discriminative ability: Table 5 shows the ability of the three hemodynamic parameters to discriminate fluid responders from non-responders, reported as area under the ROC curve (AUC). PPV had the highest AUC (0.829) followed by CVP (0.40) and PAOP (0.213). A study by Soliman et a.l21 showed that a PPV > 10.2 predict responders with a sensitivity of 92.9% and specificity of 90.9% (AUC 0.974). A study by Grassi et a.l3 investigated the capacity of PPV in predicting fluid responsiveness in patients with spontaneous breathing, and noted that PPV had an AUC of 0.87. The AUC of PPV in this study was lower compared to that of Soliman and Grassi, but still it is superior to that of PAOP and CVP and their differences were statistically significant (p<0.0001). It is plausible that CVP had a higher discriminative ability than PAOP because the cut-off of PAOP in this study may still be a little high (<12 mmHg). In summary, PPV (a dynamic parameter) has a higher discriminative ability to identify fluid responders from non-responders when compared with CVP and PAOP (static parameters of filling pressure).23
Limitations: The applicability of this study is limited only to patients who has no factors that may impair the correct interpretation of PPV (spontaneous breathing, open chest after surgery and sustained arrhythmia e.g atrial fibrillation), as well as those without conditions that make determination of cardiac output measured via Td erroneous (residual severe regurgitant lesions, and very low cardiac output).
The hemodynamic parameters that independently predict fluid responsiveness include a low baseline CVP (CVP ≤ 6 mmHg), low baseline PAOP (PAOP ≤12 mmHg), PAOP increase by ≥ 7 mmHg, high baseline PPV (PPV >12%), and PPV decrease by >5%. In addition, weight also independently predict fluid responsiveness. Among the hemodynamic parameters, PPV >12% had the highest sensitivity (92%) and PPV decrease by >5% had the highest specificity (79%) among the preload indices; while PAOP ≤12 mmHg had the lowest sensitivity (70%) and CVP ≤6 mmHg had the lowest specificity (51%). PPV also had the highest ability to discriminate fluid responders from non-responders (AUC 0.83) compared to PAOP (AUC 0.21) and CVP (AUC 0.40) (P<0.0001).
Dynamic measure of filling pressure (represented by PPV) is superior to the traditional static measures of filling pressure (PAOP and CVP) in terms of accuracy and discriminatory ability in predicting fluid responsiveness in the immediate post-operative period of cardiac surgery. PPV is the favored monitoring tool as guide to initiate fluid therapy in this clinical setting. However, in the absence of PPV monitoring, PAOP and CVP may still be used to guide fluid therapy although they are less accurate than PPV (Appendix).
Based on the findings of this study, we recommend that a high PPV (>12%) and a decrease of PPV by >5% in response to fluid challenge as the parameters to guide initiation of fluid therapy in this clinical setting. However, in the absence of PPV monitoring, or the development/ presence of conditions that may impair proper interpretation of PPV (i.e. spontaneously breathing or awake patient, open chest or atrial fibrillation), a low CVP (≤6 mmHg), a low baseline PAOP (≤12 mmHg), and an increase in PAOP ≥ 7 mmHg in response to fluid challenge may also be considered although these have lower accuracy than PPV.
Although Td remains as the gold standard measure of cardiac output, it may have its practical drawbacks. We recommend doing a similar study using a less invasive measurement techniques (i.e. PiCCO plus or EV1000) because not all patients that need hemodynamic measurement have the complete invasive set-up (pulmonary artery catheter system capable of the Td technique). Also, these less invasive techniques are capable to monitor CO more frequently (if not continuously), thereby being able to see the changes of the SV and CO in response to fluid challenge real time. Also this study did not include those patients with circulatory collapse, such as septic shock or cardiogenic shock. Making a similar study involving these patients may be important in determining the value of fluid responsiveness testing in these clinical settings. A local study comparing other dynamic parameters such as stroke volume variability (SVV) and passive leg raising (PLR) may be considered as well to see which dynamic parameter is superior in these various clinical settings.
The thermodilution (Td) cable needed for cardiac output monitoring was borrowed from Variance Trading Corporation who are also the distributor of Nihon Kohden cardiac monitors. The company has agreed to lend one Td cable at no expense. This company has no direct role in the conception, conduct, analysis and publication of this study. Furthermore, the authors/investigators have no financial conflicts of interest to disclose.
BA: conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, collection and assembly of data.
LH: conception and design, analysis and interpretation of the data, administrative, critical revision of the article for important intellectual content technical and logistic support.
The authors would like to thank their colleagues, friends and family who in one way or another have contributed for the improvement of this paper.

©2021 Albano, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.