Journal of
eISSN: 2373-6437


Literature Review Volume 15 Issue 6
1Anesthesiologist, first-year resident in the neuroanesthesiology subspecialty, Manuel Velasco Suarez National Institute of Neurology and Neurosurgery, UNAM Faculty of Medicine, Mexico
2Anesthesiologist, first-year resident in the neuroanesthesiology subspecialty, Manuel Velasco Suarez National Institute of Neurology and Neurosurgery, UNAM Faculty of Medicine, Mexico
3Anesthesiologist, first-year resident in the neuroanesthesiology subspecialty, Manuel Velasco Suarez National Institute of Neurology and Neurosurgery, UNAM Faculty of Medicine, Mexico
4Medical specialist in internal medicine, specialty in endocrinology and high specialty in thyroid diseases, San Angel INN University, Mexico
Correspondence: Gema Lizbeth Jiménez Velasco, Anesthesiologist, first-year resident in the neuroanesthesiology subspecialty, Manuel Velasco Suarez National Institute of Neurology and Neurosurgery, UNAM Faculty of Medicine, Mexico, Tel 3334957492
Received: November 01, 2023 | Published: November 13, 2023
Citation: Velasco GLJ, Rizo BN, Guerrero OO, et al. Paragangliomas, strategies in transanesthetic management. J Anesth Crit Care Open Access. 2023;15(6):161-165. DOI: 10.15406/jaccoa.2023.15.00573
Paragangliomas are rare neoplasms that usually go unnoticed or are discovered due to compression of adjacent structures or due to clinical changes secondary to the production of catecholamines depending on the embryological origin of the tumor. The diagnostic approach through clinical symptoms, biochemical tests, and imaging studies is of utmost importance to identify the tumor origin, location, invasion of adjacent structures, and compromise of the vasculature; This allows staging the tumor and taking pre-surgical measures to improve the trans-surgical-anesthetic conditions that offer a reduction in morbidity and mortality as well as the risk of complications. The search was carried out in Pub Med , World Wide Since , SciELO, Redalyc, etc. (2010-2022) using different MeSH terms , including “paragangliomas”, “nasal paragangliomas”, “catecholamines”, “pre-surgical management”, “transoperative management”. The Last search was carried out in September 2022, where a total of 29 articles were obtained, from which the information on paragangliomas and strategies in anesthetic management was synthesized.
Keywords: paragangliomas, pheochromocytomas, preoperative management, alpha-adrenergic blockade, transanesthetic management
Paragangliomas are benign neoplasms and rarely present malignant tissue; they originate in paraganglia and extra- adrenal tissue. They are small groups of neuroendocrine cells that arise from ganglia in the autonomic nervous system, these are embryologically subdivided into two subgroups: sympathetic and parasympathetic paragangliomas; The difference lies in the type of tissue from which they are formed, hormonal production, and location. The most frequent sympathetic origin is in the thorax and pelvis while parasympathetic origin predominates in the head and neck.1,2
The incidence of these injuries is low but their importance is due to the potential complications that they can trigger, since early diagnosis and management represent an opportunity for cure. The vast majority require surgical approaches which have significant morbidity rates.2
The purpose of this review is to conduct a bibliographic search to understand the pathological entity and recommendations for pre-and trans-surgical anesthetic management in adult patients with paragangliomas.
The search was carried out in Pub Med , World Wide Since , SciELO, Redalyc, etc. (2010-2022) using different MeSH terms , including “ paragangliomas ”, “paragangliomas” nasals”, “catecholamines”, “pre-surgical management”, “transoperative management”. The Last search was carried out in September 2022.
Definition and incidence
Paraganglionas arise from nerve cells of any paraganglionic system derived from pluripotent stem cells of the neural crest of the autonomic nervous system.1,2 Head and neck paragangliomas originate from the parasympathetic system, their production of catecholamines or the probability of metastasis is very low and they usually have a local invasive nature. It is suspected that the growth of nasal paragangliomas is influenced by persistent paraganglionic tissue in the maxillary artery which physiologically degenerates at birth. Paragangliomas in the thorax, abdomen or pelvis in which sympathetic tissue predominates are more common and tend to be chromaffin and secrete catecholamines such as epinephrine and norepinephrine 12% and 85% respectively.2,3–5
Paragangliomas are rare neoplasms with an incidence of 0.8 per 100,000 people per year; They can go unnoticed and be discovered postmortem.3 Head and neck paraganglioma statistics according to the WHO represent 0.6% of all tumors and the clinical incidence is 1/100,000 patients per year with a predisposition for the female sex. The most frequent locations are the carotid bifurcation, jugular foramen, vagus nerve and middle ear.4 It is worth mentioning that sinonasal paragangliomas are extremely rare and the literature reports less than 40 cases.1,5
Risk factor's
There is an increase in cardiovascular risk due to the tumor's deelete own production of catecholamines, which cause cardiac dysfunction; In addition, the decrease in circulating volume causes transsurgical hemodynamic instability, arrhythmias, and hydroelectrolyte disorders. Non-catecholaminergic tumors can compress adjacent structures and depending on the location, this is the triggering risk factor.6
Physiopathological bases
The pathophysiological basis focuses on the origin of tumor cells; tumors with sympathoadrenal tissue are capable of synthesizing biochemically active compounds such as epinephrine and norepinephrine. Unlike organs innervated by the sympathetic system, where the release of these active compounds is mediated by central neural influence, these tumors seem to lack it, probably because this anomalous tissue is not innervated.7 According to the WHO, they are classified as follows (Table 1).
Tumors of the adrenal cortex cortical carcinoma |
Tumors of the adrenal medulla and extra-adrenal paraganglia |
cortical adenoma |
Pheochromocytoma |
Sex cord stromal tumors |
Head and neck paraganglioma |
Granulosa cell tumor |
Sympathetic paraganglioma |
Leydig cell tumor |
Neuroblastic tumor of the adrenal gland |
adenomatoid tumor |
Neuroblastoma |
Mesenchymal and stromal tumors |
Nodular ganglioneruoblastoma |
Myelolipoma |
Mixed paraganglioneuroblastoma |
Schwanoma |
Ganglioeuroma |
Hematological tumors |
Composite pheochromocytoma |
Secondary tumors |
Composite paraganglioma |
Table 1 Modified WHO classification of tumors of the adrenal glands and extra-adrenal paraganglia
Classification according to the WHO8
Clinical manifestations
The symptoms of paragangliomas are diverse and can be divided into those that secrete catecholamines and those that do not (Figure 1). Secretory (sympathetic) paragangliomas present with paroxysmal symptoms that can be spontaneous such as: headache, tachycardia, diaphoresis, nausea, hypertensive crisis, acute myocardial infarction and heart failure. They can also be triggered by factors such as: exercise, medications (glucocorticoids, ephedrine), stress, alcohol and cheese.6,7
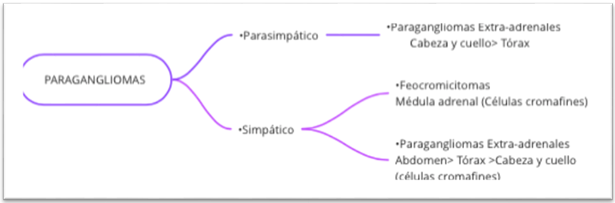
Figure 1 General description of pheochromocytomas and paragangliomas by function, location and cell type.
Non-secretory (parasympathetic) paragangliomas are most often discovered by incidental imaging studies or symptoms of compression of adjacent structures with nerve involvement, hearing loss, tinnitus, dysphagia, cranial nerve palsies, etc7,8 Specifically, sinonasal paragangliomas often present with recurrent episodes of nasal obstruction, facial edema, frontal headache, and loss of smell.1
Diagnostic approach
15-40% of catecholamine-producing paragangliomas and 30% of non-catecholamine-producing paragangliomas are associated with genetic syndromes. Multiple related oncogenes have been described, the most prevalent being SDHB, SDHD, VHL, RET and NF1, therefore part of the diagnosis includes a genetic assessment9,13 ; Biochemically, the first- line study for identifying the type of paraganglioma is the measurement of metanephrines in plasma and 24-h urine. The plasma test has a high sensitivity of 90-100% with a specificity of 79-100%, the urine test has been slightly lower in both sensitivity and specificity. False positives may occur in 19-21% of cases, triggered by the interaction with medications such as phenoxybenzamine, tricyclic antidepressants, paracetamol, labetalol, alpha-methyldopa, MAOIs and levodopa, which must be adjusted before the test.9
Another important laboratory study is chromogranin A, secreted by several types of paragangliomas, its sensitivity is around 98% with a specificity of 97%; Therefore, the best combination for the diagnosis of paraganglioma is obtained by testing metanephrines and chromogranin A in plasma.10,11
The imaging studies used in the case of paragangliomas are the following: magnetic resonance imaging, which is used to determine location, invasion of adjacent structures, vascular infiltration and metastasis; the latter shows a characteristic salt and pepper pattern secondary to tumor vascularization. Computed tomography is helpful in delineating damage to bone structures. Currently, angiography is positioned as the ideal imaging method to define the vascular anatomy, identify the blood supply of the tumor and allow preoperative embolization to be performed, thus reducing intraoperative bleeding. Functional imaging such as positron emission tomography (PET) or scintigraphy facilitates the detection of tumors when there is a possibility that they may be multiple, such as in hereditary diseases.7,12,13
Surgical treatment
Surgical resection is the cornerstone in the treatment of this pathology and is a phase I of management since it is the only curative modality. The objective is to achieve a complete resection of the tumor, as well as the affected adjacent organs, the surgical technique will be individualized and the decision-making must consider the type of tumor since if it is multicentric or malignant tumors in advanced stages, it can be opt for observation strategies and alternative treatments. Radiotherapy is a non-invasive option, indicated in high surgical risk locations. Currently, stereotactic ablative radiotherapy has achieved tumor control rates of 90-100% and symptomatic improvement in 80% of patients. Radiometabolic therapy is an option in patients with metastatic or locally advanced disease; however, considerations related to the toxicity of radiometabolic treatment must be taken, among which toxicity to the bone marrow, kidney, and thyroid stands out.14
Preanesthetic assessment
Perioperative strategies are aimed at 3 fundamental aspects: (a) treatment of hypertension and arrhythmias (b) recovery of intravascular volume and (c) treatment of secondary pathologies associated with excess catecholamines.15 The associated comorbidities cause organ damage and lead to the manifestation of pathologies such as: hypertension, Takotsubo syndrome, myocarditis, dilated cardiomyopathy, coronary syndromes, secondary pulmonary edema, thromboembolism, hypoglycemia secondary to increased hepatic glycogenolysis and inhibition of insulin secretion and retinopathy.16,17 Therefore, a complete hormonal, radiological and cardiological evaluation is necessary.8 Pre- surgical pharmacological therapy is focused on the administration of alpha and beta-adrenergic blockers, the first choice being phenoxybenzamine, a non-selective α blocker at a dose of 10 mcg/12h up to a total dose of 1 mg/kg, which must be started at least 7 to 14 days before surgery to reduce the risk of organ damage secondary to excess catecholamines.18
Other alpha adrenergic antagonists include prazosin, and terazosin at doses of 2-5 mg two to three times a day.7,19,20 Beta blockade is especially important for patients with epinephrine-secreting tumors that present with tachycardia or arrhythmias, which ideally begins four days before the surgical procedure and short-acting oral medication such as metoprolol is preferred; However, special care must be taken in asthmatic patients or patients with cardiac pathology due to the risk of developing pulmonary edema or congestive heart failure.11,21 Therefore, part of the preanesthetic evaluation should include a 12-lead electrocardiogram to detect signs of ischemia, ventricular hypertrophy, and arrhythmias.22 An echocardiogram should also be performed in patients who have a long history of high blood pressure. Patients with diabetes have been associated with greater fluctuation in preoperative stroke volume, which has been related to worse postsurgical outcomes; Therefore, they must be considered in preanesthetic planning.23
It is common to find hyperglycemia and hematocrit above 45% in presurgical clinical studies, which is associated with a significant reduction in circulating volume in relation to the increase in serum catecholamines. These values tend to improve after alpha and beta blockade.24,25
The primary objectives for the treatment of catecholamine-producing tumors are to normalize heart rate, restore cardiac volume and reduce the risk of catecholaminergic storm, therefore the objectives of preoperative treatment must meet the Roizen criteria (Table 2).
Recommended anesthetic medication |
1. Avoid atropine since its vagolytic effect can enhance catecholamine-induced tachycardia. |
2. Avoid phenothiazines in premedication as they enhance the antiandrogenic effect. |
3. Avoid droperidol as it can trigger hypertension by inhibiting catecholamine reuptake and releasing |
4. Do not use histamine-releasing substances, as they release catecholamines from chromogranin granules. |
5. Do not use ketamine for induction of anesthesia. Some authors prefer etomidate because it guarantees |
6. Regarding neuromuscular relaxants, the use of vecuronium/rocuronium is recommended since they have |
7. Sevoflurane has few cardiac effects and vasodilatory effects. |
8. Using fentanyl, alfentanil, or remifentanil instead of morphine. |
9. In cases of locoregional anesthesia, it must be taken into account that it can enhance the response of the |
10. Anxiolytic drugs, such as midazolam, should be administered before induction of anesthesia. Additionally, |
Table 2 Recommended anesthetic medication
Araujo Castro and collaborators issue the following recommendations13
Roizen criteria are used to measure the effectiveness of preoperative α- blocking.20
Roizen criteria:
Anesthetic Management
An essential part of management is having adequate monitoring, invasive blood pressure, electrocardiography, oxygen saturation, capnography, temperature, monitoring of anesthetic depth and, if possible, transesophageal echocardiography; Additionally, it is suggested to have a central venous catheter for the administration of vasoactive drugs. It is imperative to have goal-guided fluid management and make use of both dynamic and static variables.19,26,27 During induction and anesthetic maintenance there are different drug alternatives, the objective is to avoid those that have significant cardiovascular alterations. There is no solid scientific evidence that shows superiority regarding balanced general anesthesia and the use of total intravenous anesthesia, however, the use of halogenated agents such as sevoflorane has shown good results in this type of patients due to its hemodynamic profile compared to other halogenated.6,11
Control of analgesia can be a challenge during the intraoperative period because classic hemodynamic changes can be masked by a release of catecholamines typical of tumor manipulation; opioids are the drugs of choice, and remifentanil is the first-line opioid in intraoperative management; Plasma concentrations can be used that reduce the production of endogenous catecholamines in the face of surgical stimulation.25
During the resection, a hypermetabolic state may occur and episodes of hyperglycemia are common; It is advisable to periodically monitor their levels, depending on the phase of surgery, since they can cause both hyperglycemia and hypoglycemia.13
There are some drugs prohibited during these procedures due to their adverse effects on the patient with increased sympathetic activity, such as: Dopamine blockers (metoclopramide) since they could induce a hypertensive crisis, glucagon has been shown to release catecholamines in this type of tumor, sympathomimetics (ketamine) due to the potential risk of producing an arrhythmia or hypertensive crisis and histamine-releasing medications (morphine, atracurium and cisatracurium).24
Hypertensive crisis
Antihypertensive treatment is one of the biggest challenges during the procedure; the main drugs are summarized in Table 3.11 Arrhythmias are not an infrequent event in this pathology; their management must be dominated by the anesthesiologist (Table 4).
Antihypertensive treatment |
|
|
Drug |
Action mechanism |
Dose |
Nitroprusside |
Arteriovenous vasodilator |
Infusion: 0.5-5 mcg /kg/min |
Phentolamine |
Non-selective blockera |
Bolus: 1-5 mg / Infusion: 0.5-1 mg/min |
Nicardipine |
calcium channel blocker |
Infusion: 5 mg/ hr increase 2.5 mg every 15 min |
Clevidipine |
calcium channel blocker |
Infusion: 1-2 mg/ hr (do not exceed 32 mg/ hr ) |
Labetalol |
Blocker aandb |
Bolus: 5-20 mg |
Esmolol |
Selective Blocker b1 |
Bolus:10-50mg IV |
|
|
Infusion: 25-250mcg/kg/min |
Table 3 Antihypertensive treatment
Sinus tachycardia or supraventricular tachycardia |
1. Β adrenergic antagonist |
1.1. Esmolol: Continuous infusion or intravenous boluses. The recommended initial intravenous dose is 50 |
1.2. Propranolol: Not recommended because it has a long half-life and is not cardioselective. |
1.3. Atenolol: More cardioselective although it does not have a short half-life and its management may be |
2. Amiodarone |
In patients with known cardiomyopathy. Starting dose 300 mg IV |
Ventricular extrasystole |
1. Lidocaine intravenous bolus at a dose of 1 mg/kg |
Table 4 Management of arrhythmias8
Management of hypotension
During phase II of surgery (after tumor resection), it is necessary to suspend the administration of vasodilators and consider starting a vasoactive drug. The first-line drugs are: Phenylephrine (bolus: 40-160 mcg / infusion: 20- 200 mcg/min), norepinephrine (0.01-0.3 mcg/kg/min), ephedrine (bolus: 5-25 mg), it is important to remember that these drugs are not indicated if incomplete tumor resection is suspected.9,16,28 Within post-surgical care, the main considerations are: recovery of adrenergic function, stabilization of blood pressure levels, avoidance of hypoglycemia and adrenal insufficiency.9,16
Paragangliomas of the nose and paranasal sinuses are slow-growing neoplasms, which have their origin in the paraganglionic system and are rare lesions.19 They have a tendency towards progressive invasion, which confers an increase in morbidity; surgical excision with disease-free edges continues to be the treatment of choice for this neoplasm. It should be noted that this entity has a high degree of recurrence due to its nature and location.28 Regarding auxiliary techniques, radiotherapy is reported in the literature with variable results; this approach is reserved for patients without surgical indications or in those with incomplete resection. As for embolization, it has been used to restrict tumor blood volume prior to surgical resection, showing good results.11,28
It has been observed in studies that mortality after resection of paragangliomas decreases from 13-45% before alpha blockade vs. 3% once this blockade is properly established, so it is concluded that pre-surgical pharmacotherapy continues to be the mainstay in the management of this pathology.20 In patients with head and neck paragangliomas derived from the parasympathetic system, alpha-adrenergic receptor blockade may not be necessary; It is recommended to evaluate with the multidisciplinary team.19,27 As a preoperative strategy, it has been described that volume expansion can be performed to minimize severe or prolonged hypotension after the surgical procedure.26
The surgical approach focuses on total resection of the lesion with free edges through minimal invasion when possible, which is the curative modality and should be planned to minimize morbidity.28 Currently, preoperative embolization is recommended in tumors larger than 3 cm to reduce the vasculature and size of the tumor, and in some specific cases radiotherapy can be offered.4 The prognosis for this condition is related to its origin and characteristics, it is reported that 97% of paragangliomas are benign and the recurrence of a head and neck paraganglioma is around 10%, the malignant transformation of the tumor is 2 -13% up to 13 years after diagnosis.4
In general, the survival rate in patients with pheochromocytoma or paragangliomas ranges between 34-74% at 5 years and treatment in patients with metastases is referred to palliative techniques.28 Preoperative assessments by both the Endocrinology and Anesthesiology services are of high importance, to categorize risks, hypertensive control when possible and optimization of blood volume; The main objective is to block catecholaminergic activity, initiating pharmacological treatment weeks prior to resection.28,29 Anesthetic management represents a challenge for anesthesiologists; it requires adequate monitoring, knowledge of the surgical phases, and use of ventilators and vasoactive drugs at the right time.3,17
None.
The authors declares that there are no conflicts of interest.

©2023 Velasco, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.