Journal of
eISSN: 2373-6437


Review Article Volume 14 Issue 4
High specialty algology service; Hospital General de México “Dr. Eduardo Liceaga, Mexico
Correspondence: Claudia I. Gutiérrez-Román, High specialty algology service; Hospital General de México “Dr. Eduardo Liceaga, Mexico City, Mexico
Received: July 21, 2022 | Published: August 9, 2022
Citation: Gutiérrez-Román CI. Molecular basis of pain: Membrane receptors involved in pain. J Anesth Crit Care Open Access. 2022;14(4):126‒130. DOI: 10.15406/jaccoa.2022.14.00522
Pain is the most commonly reported symptom by both adult and pediatric patients, the International Association for the Study of Pain (IASP) defines pain as "An unpleasant sensory and emotional experience associated with, or similar to that associated with, actual tissue damage or potential. Pain can have a significant negative impact on quality of life in the context of human disease, in temporality physiological pain is usually acute and pathological pain is usually chronic. Nociceptors are specialized nerve fibers for pain, they are found in the skin, organ of movement, they are classified as myelin fibers that are called Aδ; and unmyelinated fibers or C fibers. Nociceptors can be stimulated by biological agents, electrical, thermal, mechanical and chemical stimuli, once the stimulus is received it will be transmitted to the brain. One of the pillars of anesthesia is analgesia, that is to say, inhibiting pain. In this bibliographic review, the aim is to explain the molecular bases, emphasizing the main membrane receptors activated in the presence of pain.
Pain is an important physiological phenomenon, evolutionarily conserved, that is necessary for survival. It is also one of the most frequent symptoms of a variety of pathological disorders and represents a significant clinical challenge. There are two basic categories of pain: physiological pain, which has an important protective function, and pathological pain, which can have a significant negative impact on quality of life in the context of human disease, in temporality physiological pain is usually acute and pathological is usually chronic. The International Association for the Study of Pain (IASP) defines pain as «An unpleasant sensory and emotional experience associated with, or similar to that associated with actual or potential tissue damage».1,2
Nociception is the neural process of encoding noxious stimuli, it is carried out specifically by nociceptors. When activated, nociceptors or Aδ and C fibers participate in the release of sensitizing substances (prostaglandins, bradykinin, serotonin, substance P, histamine, cytokines, etc.), all of them ligands of various membrane receptors. The pain is transmitted and reaches the dorsal horn of the spinal cord, from there the information is sent in 2 directions: back to the periphery, starting a withdrawal reaction (autonomous consciousness) and simultaneously to the thalamic region and other regions that function as relay pathways to the cerebral cortex. During the pain response, the nervous system undergoes a polarization of the excitatory nervous system. On the other hand, depending on their location and characteristics, nociceptors can have specific functions, which is explained molecularly by the expression of both inotropic and metabotropic receptors in specific cells and situations.3,4 This chapter briefly describes the molecular bases of pain. It is important to mention that in chronic pain, a biomolecular deregulation has been demonstrated, represented by gene silencing and activation, for which nociceptors may have changes in the expression of receptors in their membrane. and this in turn favors the presence of allodynia, hyperalgesia, dysesthesia, etc.
Nociceptors: translators of pain
Nociceptors serve as sensors for strong mechanical stimuli, noxious chemical and thermal irritants, i.e. stimuli that are capable of causing tissue injury can reach the threshold to activate nociceptive nerve endings and generate action potentials, which are then transmitted and perceived as action signals. pain. The nociceptors participate in the transduction of pain, that is, they transform a painful stimulus into an electrical one so that it can be transmitted to the dorsal up of the spinal cord (mainly in lamina I, II and V), in this place the tracts are formed. lateral spino-thalamic that is related to the sensory and discriminative part, that is, when it reaches the cerebral cortex it allows the interpretation of pain; and the spinoparabrachial tract, which will have the function of giving an emotional and/or aversive response to pain (Figure 1).5,6

Figure 1 Nociceptive sensory detection and transmission in the ascending pain pathway (modified from Sun ZC.et.al. 2020).
Nociceptors detect external pain stimuli through a variety of receptors which transduce the signal for transmission to the spinal cord and central nervous system, leading to sensory discrimination and affective pain motivation (interpretation).
There are two main types of nociceptors that vary in thickness and myelination. On the one hand, we have the Aδ fibers that are myelinated and that are responsible for transmitting fast, sharp and well-localized pain; while the "C" or unmyelinated fibers of small diameter that transmit a slow and poorly localized pain. There are also silent nociceptors that respond more sensitively to chemical stimuli (eg, capsaicin and histamine) but do not respond mechanically unless preceded by tissue injury.4 Nociceptors express various receptors on their cell membrane, mainly ionic, that allow the detection of stimuli with the potential to cause damage. When a noxious stimulus activates a receptor on a nociceptor, neurotransmission is activated and therefore pain can be perceived. But, how can we feel pain due to thermal, chemical and other stimuli? This is possible thanks to the fact that nociceptors express a great diversity of receptors on their cell membrane, each one of them with specific functions. Although in vivo nociceptors have been shown to be unimodal, most of them report polymodal functionality. This is explained by the fact that it has been observed that they become more sensitive to painful stimuli, which favors their differentiation into polymodal neurons. On the other hand, RNA analysis has shown that each nociceptor has its own transcriptome, this means that they may or may not express various ion channels and this in turn influences their response. We must take into account that this will include molecular polymorphisms, point mutations that can alter the response to nociceptive stimuli, for example, it has been seen that mice that lack NaV 1.7 channels and NaV 1.8 remain do not usually have alterations in pain perception intestinal, but if they can present greater headache.3,5,6
A-δ nociceptors, despite being polymodal, can be subdivided into Type I and Type II. Type I A-δ nociceptors function to respond to mechanical and chemical stimuli, and detect heat only at high thresholds (greater than 50 degrees C). In contrast, Type II A-δ nociceptors have a much higher sensitivity to heat, but possess a very high mechanical threshold. Likewise, C fibers are polymodal and, therefore, respond to both mechanical and thermal noxious stimuli (Table 1).4,7
Fiber |
A-δ |
C |
Diameter |
2-5 μm |
‹2 μm |
Driving speed |
5-15 m/s |
0.5-2 m/s |
Distribution |
Body surface, muscle and joints |
Other tissues (viscera) |
Synapse position within the dorsal horn of the spinal cord |
Lamina I y V |
Lamina II |
Pain cessation |
Fast, well localized, stabbing and sharp |
Slow, diffuse, dull and chronic |
Table 1 Primary characteristics of nociceptors
Receptors involved in pain perception
The expression of both metabotropic and inotropic receptors on the surface of neurons, both first-order (nociceptors) and second-order and at the brain level, are responsible for giving specificity to the physiological response to painful stimuli.
Metabotropic receptors involved in pain perception
Muscarinic receptors, mainly M1 and M2, profoundly regulate nociceptive transmission at the spinal cord level through pre- and post-synaptic mechanisms. Elevation of Ach levels in the spinal cord induces analgesia, whereas local decreases in Ach levels or activity (via receptor blockade) potentiate nociceptive sensitivity, inducing hyperalgesia and allodynia. It has even been shown in ancient times that acetylcholinesterase (AchE) inhibitors administered intrathecally, such as neostigmine, reduce inflammatory hypersensitivity attributed mainly to the activation of the M2 receptor, as it is the main cholinergic receptor expressed in the spinal cord. On the other hand, an overexpression of M4 has been demonstrated in the presence of neuropathic pain.8
Inotropic receptors involved in pain perception
Transient receptor potential (TRP)
Transient receptor potential (TRP) is a superfamily of receptors subdivided into seven subfamilies, 6 of which are expressed in humans:(Figure 2)
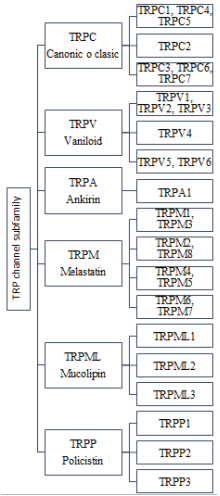
Figure 2 Superfamily of TRP channels expressed in humans. We observe in order the subfamilies that have been named based on their time of discovery, as is the case of TRPC channels and based on their main ligand, then the isoforms of each one are classified based on their structural homology.
All this superfamily has in common, that they are proteins composed of 6 transmembrane segments (S1 to S6), a region that forms a pore (P) between the transmembrane segments S5 and S6 and intracellular carboxyl and amino terminals. TRP channels have a wide tissue distribution, including: smooth muscle, epithelium, immune cells, skeletal muscle, heart, and neurons. They constitute cellular sensors for a wide spectrum of mechanical, chemical and physical stimuli; but it also participates in other organic functions such as smell, taste, etc.5,9
There are only ten thermosensor TRP channels, which are characterized by activation at different temperatures. There are TRPV1–4, TRPM2, TRPM4, and TRPM5 channels that are activated by heat and TRPM8, TRPA1, and TRPC5 that are activated by cold temperatures. These channels are also activated by mechanical and chemical stimuli, including both plant-derived and environmental (exogenous) compounds, as well as endogenous inflammatory molecules such as serotonin, bradykinins, prostaglandins, proteases, substance P, and growth factors. Thermal stimuli can be broadly classified into hot pain, harmless heat, harmless coldness, and cold pain. The average thresholds for detection of harmless heat or cold are between 1.3 to 6.2 °C above and 0.9 to 3.4 °C below skin temperature, respectively. While the average detection threshold for painful heat is between 41.5 and 47.0 °C and for painful cold it is between 7.3 and 18.4 °C10.
TRPV1, is a non-selective cation channel, is the most investigated and its fundamental role in the detection of noxious stimuli. It is a polymodal channel that can be activated by heat at 43°C or more and at the same time it can be activated by vanilloids (capsaicin, anandamide, etc), vanillotoxins and protons, it is expressed almost exclusively in thin or medium caliber C fibers.5,6 Melastatin transient potential receptors TRPM has 8 isotypes, each with 2 isoforms and have been called TRPM 1-8, they collectively regulate the fluxes of several types of cations such as K +, Na +, Ca 2+ and Mg 2+. TRPM2 is a heat-activated Ca+ channel that has been shown to participate in inflammatory activation during acute pain, predisposing to chronic pain after nerve injury in a murine model. TRPM3 is a protein expressed in the dorsal horn ganglia and in the trigeminal ganglion, the current results relate it not only as part of the still unresolved mechanisms of chronic pain, but also has the potential to become a clinically relevant pharmacological target of future analgesic strategies. TRPM8 is activated by both cold stimuli and a variety of synthetic cooling agents, such as mentol.10–13
Transient receptor potential Ankyrin 1 (TRPA1), is an ion channel expressed predominantly in a subset of nociceptive somatosensory neurons, specifically in C fibers where it acts as a polymodal sensor for various physical and chemical products. Stimuli of extracellular or intracellular origin. This channel is characterized by a marked promiscuity of the activating agent towards painful or potentially harmful stimuli, including natural and exogenous electrophilic compounds, ultraviolet radiation, oxygen, extreme temperatures, chemical irritants, and endogenous inflammatory mediators. Studies with TRPA1 knock-out mice and specific antagonists have linked the function of TRPA1 to the regulation of temperature perception, inflammation, mechanical sensation, pain, itching, but also allows homeostasis between the nociceptive and the immune system.14
TRPC channels act as a direct mechanical sensor, that is, it is possible that they are necessary to regulate intracellular calcium levels, this in turn modulates the activity of other direct mechanical sensors, although this possibility is still an area to be investigated. TRPC3 and TRPC6 have been shown to be essential for mechanotransduction in subsets of sensory neurons and cochlear hair cells. While TRPC1, TRPC5 and TRPC6 are sensitive to membrane stretching, which translates their importance in mechanical stimuli. The homology of these channels allows them to compensate for the absence of TRPC3 and TRPC6, although this is not always the case and could result in hearing impairment or vestibular deficit. TRPC3, TRPC 4 and TRPC 5 increase in both transcript and protein in the presence of nerve injury, gradually decreasing, returning to normal after 15 days and up to 3 months. In addition, TRPC1, TRPC3, and TRPC6 have also been upregulated in the spinal cord of mice that have morphine analgesic tolerance and hyperalgesia after chronic morphine exposure (Table 2).6,15
Table 2 Main receptors proposed for noxious cutaneous stimuli (modified and translated from Dubin A and Patapoutian A, 2010)
A-MH fibers (heat-activated mechanosensitive alpha fibers), C-MH fibers (heat-activated mechanosensitive C-fibers), C-H fibers (heat-activated C-fibers)
Acid sensing ion channels (ASIC)
Acid sensing ion channels (ASIC) are preferably Na+ channels, due to their 20 to 25% homology with these channels, however, they can also be permeable to Ca++. In mammals there are 4 genes that code for 6 receptors (ASIC1-4) (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4) (10). They are characterized by being expressed in muscular sensory neurons and open when activated by low pH, even ASIC1a and ASIC 3 have been associated with activity-induced hyperalgesia (mechanical hyperalgesia), showing that the inhibition of ASIC1a reduces the presence of ASIC1a, proposing as a therapeutic target. This action can be related to their activation speed, since this is fast in the case of ASC1a, ASIC 1b and ASIC 3, while ASIC2a and ASIC2b have a slower activation current. The pH level is also related to its activation of each receptor isotype, we emphasize that the pH is modified during tissue injury, observed at pH 6.5 during injury, pH 5.5-7.0 during inflammation, pH 5.8-7.4 during swelling and less than 7.1 during ischemia (Table 3).15-18
Isotype/Isoform |
pH required for activation |
Times when it can be activated |
|
ASIC1 |
ASIC1a |
6.2-6.8 |
Tissue injury, ischemia, strenuous exercise |
ASIC1b |
5.1-6.2 |
Inflamation, tumefaction |
|
ASIC2 |
ASIC2a |
4.1-5.0 (in heteromeric form with ASIC1a decreases its sensitivity to pH up to 3.9) |
Activation at very low pH (acid damage) |
ASIC2b |
It is not homomerically activated and its heteromeric form is frequently with ASIC1a. |
It has been considered that they are receptors that do not depend on pH by themselves. |
|
ASIC3 |
6.2-6.7 |
Inflammation, swelling and ischemia |
|
Table 3 PH-dependent activation of ion receptors for acid detection (ASIC)
Although ASICs can be found in the intestine, liver and other tissues. The main anatomical sites of expression are the central and peripheral nervous systems. Even ASIC1b, ASIC2b, and ASIC3 are widely expressed in small and medium nociceptive neurons; and while ASIC2a and ASIC3 are mainly expressed in medium and large sensory neurons. Furthermore, ASIC1a, ASIC2a, ASIC2b and ASIC4 are widely expressed in the brain.19
Other participating channels are:
To know in detail the receivers mentioned, we suggest you consult the previous chapters.
The expression of all the receptors activated during nociception is favored by the "inflammatory or algogenic soup" that comprises a variety of signaling molecules that act as ligands of metabotropic receptors, mainly coupled to Gq protein, such as bradykinins, prostaglandins, histamine, activating factor of platelets and ATP, which can activate their corresponding receptors located in nociceptors and cause activation in the expression of other metabotropic and inotropic receptors that participate in the transmission of pain, this mainly due to their participation in the release of Ca+, in such a way that during The nociceptive process creates a positive regulation favored by the activation of TRP channels (Ca+, Na+ channels), culminating in a potentiated activity that will not only allow the organism nociception but will also initiate the necessary signaling for the activation of immune cells that will have as main objective or the repair of the damage, although in certain cases this can be associated with the development of chronic pain, but that is another topic that is not touched on in this chapter.
The main receptors involved in pain are mainly of the ionotropic type, of which TRP, ASIC and Kv, perhaps the most important, are capable of participating in various painful sensations such as pain due to heat, cold, changes in pH, etc. Most of these receptors can be overexpressed in a painful process and participate in the development of hyperalgesia.
None.
None.

©2022 Gutiérrez-Román. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.