Journal of
eISSN: 2373-6437


Research Article Volume 5 Issue 4
Department of Anesthesiology, Aesthetic Associates Center, University at Buffalo, USA
Correspondence: Rose Berkun, President Elect, New York State Society of Anesthesiologists Clinical Assistant Professor, Department of Anesthesiology, Jacobs School of Medicine and Biomedical Sciences, The State University of New York at Buffalo, USA
Received: July 27, 2016 | Published: August 24, 2016
Citation: Berkun R, Khechen B, Berkun R (2016) Incident Reduction of Postoperative Nausea and Vomiting (PONV) Following Office-Based Surgery - A Retrospective Chart Analysis. J Anesth Crit Care Open Access 5(4): 00197. DOI: 10.15406/jaccoa.2016.05.00197
Modern PONV risk research began in the 1990s with publication of studies using logistic regression analysis to simultaneously identify multiple independent PONV predictors and publication of meta-analyses and systematic reviews. This literature shows that female gender post-puberty, nonsmoking status, history of PONV or motion sickness, childhood after infancy and younger adulthood, increasing duration of surgery, use of volatile anesthetics, nitrous oxide, large-dose neostigmine and intraoperative or postoperative opioids are well established PONV risk factors. Conflicting risk factors have been published by different studies and include history of migraine, history of PONV or motion sickness in a child's parent or sibling, better ASA physical status, intense preoperative anxiety, certain ethnicities or surgery types, decreased perioperative fluids, crystalloid versus colloid administration, general versus regional anesthesia and sedation, balanced versus total IV anesthesia, and use of long acting and short acting opioids (Table 1).1 According to a randomized controlled trial in over 5000 patients, the use of a short acting opioid remifentanil instead of fentanyl did not decrease the incidence of PONV suggesting that any opioid can trigger nausea and vomiting chemoreceptors.2 Avoidance of intraoperative and postoperative opioids has been shown to reduce PONV.3
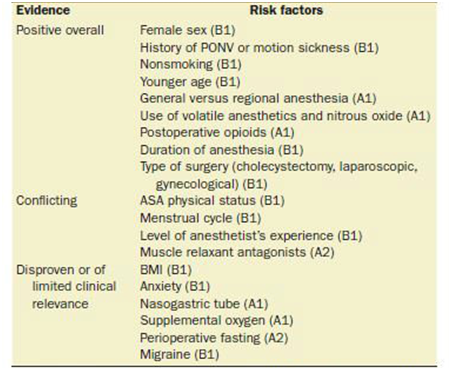
Table 1 Risk factor for PONV in Adults. PONV: Postoperative Nausea and Vomiting; BMI: Body Mass Index; MS: Motion sickness.
According to Society for Ambulatory Anesthesia 2014 Guidelines for PONV 5 risk factors were identified (Table 2) and recommendation on assessment and prevention of PONV were published (Table 3).4 Current consensus guidelines denote that patients with 0-1 risk factors still have a 10-20% risk of encountering PONV, but do not yet advocate routine prophylaxis for all patients with 10-20% risk.5 The pathophysiology of PONV is complex and not perfectly understood. According to our current model, the brain structures involved in the pathophysiology of vomiting are distributed throughout the medulla oblongata of the brainstem including chemoreceptor trigger zone (CRTZ) and the nucleus tractus solitarius (NTS) and not centralized in an anatomically defined ‘vomiting centre’.6 Multiple neurotransmitter pathways in both brain and the gastrointestinal tract are implicated in causing nausea and vomiting.
Enterochromaffin cells in the gastrointestinal tract release serotonin and the vagus nerve communicates with the CRTZ via 5-HT3 receptors. The CRTZ communicates with the NTS primarily via dopamine-2 (D2) receptors. The vestibular system, which detects changes in equilibrium, communicates with the NTS via histamine-1 (H1) and acetylcholine (mACh). Anticipatory or anxiety-induced nausea and vomiting appears to originate in the cerebral cortex, which communicates directly with the NTS via several types of neuroreceptors.7
Many studies have been conducted to evaluate the efficacy of different antiemetic agents on PONV prophylaxis. Patients who received maintenance anaesthesia with propofol had a significantly lower incidence of postoperative nausea and vomiting in comparison with inhalational agents regardless of induction agent, choice of inhalation agent, presence/absence of nitrous oxide, age of patient or use of opiate.8 General anaesthesia is associated with an 11-fold increased risk of PONV as compared to regional anesthesia. When general anaesthetic is required, the use of propofol as the induction agent is effective in reducing early PONV incidence when compared with other induction agents.9 Neostigmine, a reversal agent for non-depolarising muscle relaxants, is associated with increased PONV, especially in large doses (>2.5mg).10 Refraining from routine usage of this reversal agent still seems to be a good measure when trying to achieve best outcomes.
Prophylactic administration of dimenhydrinate is as effective as the use of ondansetron in preventing PONV in patients undergoing elective laparoscopic cholecystectomy. Dimenhydrinate is the preferred drug because it is less expensive. With more than 500,000 laparoscopic cholecystectomies performed in the United States each year. The potential drug cost savings from the prophylactic administration of dimenhydrinate instead of ondansetron exceed $7.25 million per year.11 In 2003, an expert panel compared all 5-HT3 receptor antagonists and agreed that there was no evidence of any difference in the efficacy and safety profiles of the different 5-HT3 receptor antagonists in the prophylaxis against PONV.12 Ondansetron, dexamethasone, and droperidol (when used for prophylaxis), each is estimated to reduce risk of PONV by approximately 25%.
Metoclopramide blocks dopamine receptors in the CTZ and vomiting centre. It also shortens bowel transit time and in high doses blocks serotonin receptors. When used in standard clinical doses of 10 mg, metoclopramide was found to be ineffective for PONV prophylaxis.13 The addition of 50 mg metoclopramide to 8 mg dexamethasone (given intraoperatively) has been shown to be an effective, safe and inexpensive way to prevent postoperative nausea and vomiting. A reduced dose of 25 mg metoclopramide intraoperatively, with additional postoperative prophylaxis in high risk patients, may be equally effective and cause fewer adverse drug reactions.14 Scopolamine is an anticholinergic that blocks emetic muscarinic receptors in the cerebral cortex. It is very effective, with a NNT of 3.8 for prevention of PONV.15
Postoperative nausea and vomiting (PONV) is defined as any nausea, retching, or vomiting occurring during the first 24-48h after surgery in inpatients. PONV is one of the most common causes of patient dissatisfaction after anaesthesia, with reported incidences of 30% in all post-surgical patients and up to 80% in high risk patients. In addition, PONV is regularly rated in preoperative surveys as the anaesthesia outcome the patient would most like to avoid.7 Prevention of PONV in plastic surgery patients is especially important, as it can lead to bleeding, suture dehiscence, produce unfortunate aesthetic results as well as dehydration, electrolyte imbalance, decreased ability of patient to care for him/herself and may result in unanticipated hospital admissions. Numerous investigations have been conducted to examine the incidence of PONV after general anesthesia. However, the use of regional anesthesia in combination with intravenous sedation, as it relates to PONV, has a limited number of studies.
This study examines the implementation of a specific protocol aimed to decrease the incidence of PONV at a Class C office based surgery center accredited by the American Association for Accreditation of Ambulatory Surgery Facilities (AAAASF) where both general anesthesia and sedation are administered.
In an effort to decrease the incidence of PONV for office-based plastic surgery, the approach had the goal to preemptively block all known receptors that trigger PONV prior to the administration of triggering agents. Although patient population at AAC was considered moderate risk (use of opiates, female gender, better ASA class) based on known risk factors for PONV, the approach to PONV prevention was based on treating every plastic surgery patient as high risk. The protocol consisted of 50 mg oral Dimenhydrinate (Dramamine), 4mg IV Ondansetron (Zofran), 25mg IV Diphenhydramine (Benadryl) and 10 mg Metoclopramide (Reglan) in the preoperative area. Patients with history of PONV received additional transdermal Scopolamine 1.5mg patch. A retrospective chart review pertaining to 288 plastic surgery patients between January 2014 and June 2016 was conducted. Gender was comprised of 253 females and 35 males with the age range between 18 and 81 and ASA class I and II. Procedures included liposuctions, breast augmentations, mastopexies, blepharoplasties, facelifts, rhinoplasties and mini abdominoplasties. Duration of surgeries ranged between 45 and 300 minutes. Each patient received 50mg oral Dimenhydrinate, 4mg IV Ondansetron, 25mg IV Diphenhydramine, 10mg IV Metoclopramide in the preoperative area. All patients received a combination of regional and local anesthesia and IV sedation with Midazolam, Fentanyl, Ketamine and Propofol in the form of continuous infusion. Dosing depended on duration of surgery and patient’s response. Patients were monitored for PONV in recovery and for the following 48 hours. Each patient was seen at the office within 24-48 hours and symptoms of PONV were recorded in the chart.
Total number of 288 patients comprised the sample population in this study. 6 patients reported PONV within 48 hours post surgey, which equaled 2.083% of the sample population. We used 30% incidence of PONV, the lowest reported incidence in general population, as a benchmark. Values were calculated based on binary data. Standard deviation was calculated using the equation SD=Px (1-P)/N (P=.3, 1-P=.7 and N=288), SD=(.3x.7)/288=.027. Z score was calculated using the equation Z=(P-M)/SD (M=.02083), Z=(.3-.2083)/.027=10.339. Z score greater than 2.58 correlates with P value less than 0.01 indicating that there is less than 1% probability the results in our study were due to random chance. The incidence of PONV in this study was 2.083% (P<.01) compared to 30% of reported incidence of PONV in general population.16
PONV is triggered through multiple receptors in the brain and the gastrointestinal tract. Recent studies have demonstrated that using more than one antiemetic is usually more effective and results in fewer side-effects than simply increasing the dose of a single antiemetic.17,18 The approach to reduction of PONV at the Aesthetic Associates Center concentrates on blocking receptors that trigger PONV prior to the administration of triggering agents, avoidance of general anesthesia and decrease in patient anxiety. Therefore, all antiemetics are administered prior to induction. Both Droperidol and Metoclopramide are D2 receptor antagonists. Droperidol has a short half life of 134 minutes. Metoclopramide is a central and peripheral D2 antagonist with half life of 5-6 hours and also sensitizes tissues to acetylcholine, stimulating upper GI tract motility. Ondansetron selectively antagonizes serotonin 5-HT3 receptors. Diphenhydramine antagonizes central and peripheral histamine H1 receptors and possesses anticholinergic properties. Thus all known receptors that trigger nausea and vomiting are presumably blocked. A multimodal approach for PONV prevention also includes the use of regional anesthesia, maintenance with intravenous sedation and emphasis on preoperative patient relaxation to decrease the level of anxiety. Side effects are taken into consideration which include QTc prolongation, which increases the risk of torsades de pointes with Droperidol and serotonin antagonists, extrapyramidal symptoms with Metoclopramide, dry mouth, visual disturbances and urinary retention with Dimenhydrinate and visual disturbances with Scopolamine.
This retrospective chart review results suggest that using multiple antiemetics prior to the administration of triggering agents, as well as avoiding general anesthesia, significantly reduces the incidence of PONV. There are several limitations to this study. While this study’s percentage of PONV incidence revealed the desired outcome of a mere 2.08% (P<.01) in comparison to the considerable national average of 30%, this study’s outcomes were not standardized to the type of surgery, duration of surgery, amount of anesthetic given, gender, smoking status and patient age. The results of the study were based on binary data examining decrease in incidence of PONV compared to general population regardless of other variables. In addition, this retrospective chart review was used to solely collect chart data without conducting patient interviews and there was no control group.
Based on the outcome of this analysis, the Aesthetic Associates Center has initiated a prospective study in order to implement the standardized incident ratio as the reliable strategy in determining the incidence of PONV that will account for said variables.
None.
The authors declare there is no conflict of interests.
None.

©2016 Berkun, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.