Journal of
eISSN: 2373-6437


Research Article Volume 7 Issue 5
1Professor, Department of cardiothoracic & Vascular Anaesthesiology NRS Medical College, India
2Assistant Professor, Department of community Medicine, India
3Senior Resident, Department of Anaesthesiology, India 4Professor & Director, Directorate General Medical Services (Army) Adjuvant General??s Branch, India
4Professor & Director, Directorate General Medical Services (Army) Adjuvant General's Branch, India
5Assistant Professor, Department of Anaesthesiology, Midnapur Medical College, India
6Consultant, Department of Anaesthesiology, AMRI Hospital, India
7Assistant Professor, Department of GI Anaesthesiology, India
Correspondence: Sampa Dutta Gupta, Professor, Department of Cardiothoracic and Vascular Anaesthesiology, Nilratan Sarkar medical College, West Bengal Medical Education Service, 42 Lake Place, 1st floor, Kolkata 700029, Tel +91 -9433834317
Received: May 28, 2016 | Published: April 5, 2017
Citation: Gupta SD, Mondal T, Bhattacharya S, Singh CAK, Maji S, et al. (2017) Effect of Starvation Times on Blood Glucose of Mother and Her Full Term Newborn before Spinal Anesthesia for Elective Cesarean Section. J Anesth Crit Care Open Access 7(5): 00276. DOI: 10.15406/jaccoa.2017.07.00276
Background: Pregnant mothers awaiting elective cesarean section often have fasting times much longer than recommended due to inadvertent delay in surgery. We aimed to ascertain changes in maternal and fetal blood glucose with the duration of maternal fasting.
Subjects and Methods: The study population comprised of Group A (n=17) liquid fasting between 2–3 hours, and Group B (n=17) liquid fasting more than 3 hours. Maternal and fetal cord blood glucose levels, Apgar scores at 1 and 5 minutes, the need for resuscitation and its correlation with newborn blood glucose level were estimated. All data analyzed statistically.
Results: Mean duration of liquid fasting in Gr. A: 2.65hrs; in Gr. B: 11.35hrs. Mean maternal capillary glucose level in Gr. A: 101 mg/dl; in Gr. B: 76 mg/dl. Mean neonatal cord blood glucose level in Gr. A: 96.94 mg/dl; in Gr. B: 73.76 mg/dl. Apgar score in Gr A at 1 min: 8.43 and at 5 min: 9.81; Apgar score at 1 min in Gr. : 7.62 and at 5 min: 9.7. Need for resuscitation in Gr. A: 11.76%; in Gr. B: 47.05%. Heel prick capillary glucose level (needed resuscitation) in Gr. A: 62mg/dl; in Gr. B: 44mg/dl.
Conclusion: The duration of fasting has significant negative correlation with maternal (–0.76) and fetal blood glucose levels (–0.73). Strong positive correlation exists between maternal and fetal cord blood glucose levels (0.97).
Keywords: prolonged fasting; elective caesarean section; maternal and fetal effects; neonatal resuscitation
Pregnant mothers awaiting elective cesarean section (C/S) are frequently delayed and fasting time is longer than recommended. Glucose turnover in fasted pregnant women is several times greater compared to demographically matched nonpregnant women. With a brief maternal fast before elective C/S there is a more rapid fall in plasma glucose concentration, moreover this fall does not initiate fetal glucose production in normal or diabetic subjects.1
During intrauterine life, the human fetus is completely dependent upon maternal supply of glucose and other fuels. After birth, the neonate needs to make transition to endogenous glucose production with only intermittent exogenous glucose delivery. Since infants have rates of glucose utilization threefold higher than those of adults in terms of body weight probably due to their large brains, high rates of endogenous glucose production is required to maintain systemic glucose balance. Because mobilizable glycogen stores are limited and feeding is intermittent, the newborn is largely dependent on gluconeogenesis for the first 4 to 6 hours after birth. Isotonic drinks to mother during labour has beneficial effect in mother and newborn due to decrease in beta–hydroxybutyrate and non–esterified fatty acid levels.
Prolonged fasting causes distress, dehydration, biochemical imbalance and hypoglycemia especially in fetus. Newborn of starved mother manifests apnea, cyanotic spells, hypothermia, hypotonia and poor feeding. Earlier investigation showed blood glucose of fetus and newborn at birth correlates with blood glucose of the mother and maternal hypoglycaemia can cause fetal hypoglycaemia.2 Blood glucose values of parturient vary with time of the last meal.3 It has been found that, the greatest incidence of hypoglycemia is seen immediately after birth .4 and carbohydrate–rich drink reduces pre–operative discomfort in elective surgical patients.5 Moreover injectable dextrose may precipitate fetal hypoglycemia due to more activation of fetal insulin.
An hospital audit with 32 women having elective C/S revealed that 20% of study women have a drink < 4hrs before elective C/S, more than half still fast > 10hsr.6 A guideline for the multidisciplinary team of 2005 Royal College of Nursing recommended that, intake of water up to 2hrs before induction of anesthesia for elective surgery is safe in healthy adults, and improves patient well–being. The volume of administered fluids does not appear to have an impact on patient’s residual gastric volume and pH compared to a standard fasting regimen. Therefore, patients may have water and other clear fluids up to 2hrs before induction of anesthesia.
With this background, this study was conceptualized to ascertain changes in the maternal and fetal blood glucose in relation to the duration of fasting. The aim is to compare the blood glucose levels of the mothers, undergoing elective C/S under subarachnoid block and her newborn, the need for resuscitation at birth.
With Institutional Ethics Committee approval and written informed consent this prospective, double blind, parallel group, study was conducted with 34 adult full term pregnant women of ASA physical status I, eligible for intake of preoperative clear fluids, according to the present recommendations by the American Society of Anesthesiologists (ASA) .7 undergoing elective C/S under subarachnoid block. All patients were put up for elective C/S following the fasting guidelines of ASA.7 Diabetic mothers, patients on hypoglycemic therapy, inborn metabolic errors, with infection, organ failure, abnormal mentation, dizziness, delirium, autonomic dysfunction, generalized or focal seizures, focal or general motor deficit, paralysis, hemi paresis were excluded.
Sample size calculation was done based upon anticipated correlation coefficient value of 0.5 for correlation between numbers of hours of liquid fast and maternal or cord blood glucose level. It was estimated that 29 subjects would be required in order to detect correlation at this level in the underlying population with 80% power and 5% probability of Type I error.
Following routine investigations as per ASA recommendation.8, subjects were selected on the basis of history of fasting duration with advice of standard preoperative ASA fasting Guidelines.7 According to the liquid fasting duration, study population was divided into two groups. Group A (n=17) subjects received preoperative isotonic clear drink between 2–3hrs and Group B (n=17) subjects received preoperative isotonic clear drink >3hrs before administration of subarachnoid block.
After a detailed pre–anesthetic assessment, intravenous access was secured using 18G intravenous cannula, all patients were premedicated with inj. metoclopramide 10mg and inj. ranitidine 50mg iv 10mins before subarachnoid block. All patients were preloaded with Ringer lactate infusion 10 ml/kg over 20mins. Monitors were applied with an automatic blood pressure (BP) cuff, ECG, and pulse–oximeter. Subarachnoid block was instituted using a 25G Whitacre needle at L3–L4 interspaces in sitting position with 0.5% hyperbaric bupivacaine 8–12 mg, thereafter patients were placed in supine position with a wedge angled 10°–15° placed under right hip to obtain a left uterine displacement. Onset of cephalad spread of analgesia was determined as loss of pin prick and block was confirmed at the level of T5–6. All patients were given O2 (3 lt/min) through nasal prongs.
Ringer Lactate 5–6 mL/kg/hr for 1hr, then 500 mL hydroxyethyl starch 6% solution were infused together until completion of surgery. >20% decrease in systolic BP (SBP) below baseline after subarachnoid block was defined as hypotension. Hypotension was initially treated with fluid resuscitation, if not responded, inj. phenylephrine was administered IV titrated. Bradycardia (<60/min) was treated with inj. atropine 0.02 mg/kg iv. Heart rate, respiratory rate, BP, SpO2 were noted preoperatively and every 5 minutes intra–operatively.
Inj. oxytocin (5–10 units) was infused slowly intravenously after delivery of baby. The APGAR scores of the newborn were recorded at 1 and 5 mins after delivery. Total duration of solid and liquid fasting time in hours was noted for each patient. Capillary blood glucose of mothers and newborn’s cord blood were estimated and need of resuscitation was recorded. Blood glucose was determined by glucose oxidase method.
End point of study
All data was entered into excel spreadsheet and analyzed using Statistical version.6 Numerical data were presented as MEAN±SD and analyzed by Students t–test. Parametric data were analyzed using one way analysis of variance test (ANOVA). Fisher’s exact test was used for categorical variables. All tests were two tailed. A p value of less than 0.05 was considered statistically significant. For neonatal resuscitation, American Heart Association Care Guidelines for Neonatal Resuscitation: 2010.9 were followed. For Apgar scoring system, recording of five objective signs pertaining to the condition of infants at birth were used. Apgar at 1 minute score is of value in assessing the need for resuscitation whereas at 5 minutes was strongly considered with infant mortality and morbidity. Capillary blood glucose (mg/dl) of newborn needed resuscitation was estimated by heel prick method.10
A total 56 term pregnant women awaiting elective C/S were needed to assess to select 17 subjects for each Group. Group A with liquid fasting duration between 2–3hrs and Group B with liquid fasting duration >3hrs. The pie–diagram (Figure 1) shows total 56 term pregnant women (77%) awaiting elective C/S with ASA fasting guideline were needed to survey prospectively to get 17 patients (23%) for each Group. Therefore, total 34 full term pregnant women awaiting elective C/S were included in this study. There were no cases of apparent or suspected pulmonary aspiration or other drink–related complications before, during or after surgery within the study population.
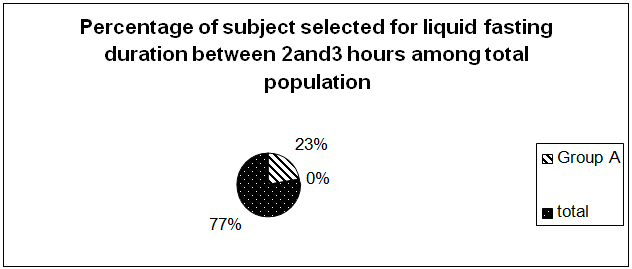
Figure 1 Shows a total of fifty six term pregnant women (77%) was required to get seventeen patients (23%) for Group A and seventeen patients (23%) for Group B for this study. Therefore, a total of thirty four term pregnant women awaiting elective C/S were assessed for study eligibility to meet the inclusion criteria.
Demographic profile (Table 1) revealed comparable results (p> 0.05) in terms of age, sex, weight, haemodynamic parameters and gestational age between the two groups. SBP (Group A 126.2±9.3; Group B 120.3±7.27), diastolic BP (Group A. 80.7±8.2; Group B. 77.62±5.62), Heart rate (Group A 104.29±14.2; Group B 92.33±9.62) before the induction of subarachnoid block were insignificant.
Table 2 shows, difference in categorical variables between the groups were done by fisher’s exact test. Analysis reveals 11.76% of neonates born to mothers in Group A neded resuscitation whereas 47.05 % of neonates born to mothers in Group B required resuscitation. Fisher’s exact test shows a p value < 0.05 which is statistically significant.
|
Characteristics |
Group A (n = 17) |
Group B ( n =17) |
P value |
|
Age (yr) (mother) |
25.24±2.22 |
24.32±2.36 |
>0.05 |
|
Weight (kg) (mother) |
58.38±2. |
55.19± 1.02 |
>0.05 |
|
Height (cm) (mother) |
151.02±1.13 |
149.242± 2.32 |
>0.05 |
|
Gestational age (wk) (mother) |
37.18±3.2 |
38.12± 2.45 |
>0.05 |
|
Hb% (mother) |
12.35±2.14 |
14.29 ± 1.42 |
>0.05 |
|
Urea mg/dl (mother) |
27.71±3.18 |
26.39 ± 1.84 |
>0.05 |
|
Creatinine mg/dl (mother) |
0.82±0.14 |
0.80±.53 |
>0.05 |
|
Prothrombin time (sec) (mother) |
13.2 ±1.22 |
12.2±0. 22 |
> 0.05 |
|
SBP (mmHg) Pre op. (mother) |
126.2±9.3 |
120.3±7.27 |
>0.05 |
|
DBP (mmHg) Pre op. (mother) |
80.7±8.2 |
77.62±5.62 |
>0.05 |
|
Volume preload (ml) (mother) |
793±23.42 |
780±25.24 |
>0.05 |
|
Heart rate /min (mother) |
104.29± 14.22 |
92.33±9.62 |
>0.05 |
|
Fasting time (hrs) Solid (mean±S.D) |
6.63±2.07 (5.53-7.74) |
14.3±5.42 (11.5-17.1)* |
<0.05 |
|
Fasting time (hrs) liquid (mean±S.D) |
2.65±0.31 (5.53-7.74) |
11.35±3.21 (9.74-12.96)* |
<0.05 |
|
Birth weight (Newborn) (mean±SD) |
2.78±0.22 |
2.71±0.20 |
|
|
1min Apgor score (mean± S.D.) (Newborn) |
8.43±1.63 |
7.625±1.644 |
<0.05 |
|
Need for resuscitation (Newborn) |
(2) 11.76% |
(8) 47.05% |
<0.05 |
|
Apgar at 1 minute |
8.43±1.63 |
7.625±1.644 |
<0.05 |
|
Apgar at 5 minutes |
9.8125 ±0.13 |
9.705±0.187 |
> 0.05 |
|
Need for resuscitation |
(2)/ 17 11.76% |
(8)/ 17 47.05% |
<0.05 |
|
Neonates with Heart rate at birth >100:100-60: <60 /min |
17:00:00 |
17:00:00 |
>0.05 |
|
Respiratory rate at birth apnoea/ labored |
Labored 2 |
Labored 8 |
>0.05 |
|
No of neonates with peripheral cyanosis |
Jan-17 |
6/17 |
< 0.05 |
|
No of neonates with central cyanosis |
0 |
0 |
----- |
|
Cord blood glucose level at birth(mg/dl) |
98 (R= 43.000) |
74.58824 (R=60.000) |
0.000113* |
|
blood glucose of Newborn (mg/dl) needed resuscitation (mean± S.D.) |
(2)/17 81.5±9.192 |
(8)/17 57.5±5.928 |
<0.05 |
|
Hypoglycemia in newborn |
0 /17 |
0 /17 |
Table 1 Demographic profile (age, sex, weight), haemodynamic parameters, gestational age were comparable (p> 0.05) between the two groups. Systolic arterial blood pressure (SBP) (Group A. 126.2±9.3; Group B 120.3±7.27), diastolic blood pressure (DBP) (Group A. 80.7±8.2; Group B. 77.62± 5.62) and values before the induction of subarachnoid block were comparable (p>0.05). Heart rate (baseline) before the induction of subarachnoid block was not statistically significant between the groups (Group A 104.29± 14.2 Group B 92.33± 9.62)
Comparison of fasting duration in hours for solid and liquid in both groups: (mean S.D.), shows that in Group A duration of liquid fasting corroborates with duration of solid fasting where fasting guidelines were strictly followed. But in Group B duration of both solid and liquid fasting is prolonged. This indicates that in Group B due to procedural delay had prolonged fasting periods (for both solid and liquid) inspite of standard fasting advice
Gestational age, birth weight and i min apgar score distribution of infants in the different fasting groups shows that, the difference between Apgar scores at 1min in Group A and Group B is statistically significant. The difference in the need for resuscitation in Group A and Group B is statistically significant
Comparison of apgar score, heart rate, respiratory rate, colour, blood glucose level in new born needed resuscitation between the groups analysed by *Mann Whitney U test, and shows a significant difference between the two groups in terms of Apgar at 1 minute, Need for resuscitation, No of neonates with peripheral cyanosis, Cord blood glucose level at birth (mg/dl) and blood glucose of Newborn ( mg/dl) needed resuscitation (mean± S.D.)
p <0.05= Statistically significant. p> 0.05 = statistically not significant.
|
Group |
Resuscitation Not needed |
Resuscitation Needed |
Row Totals |
|
|
Count |
A |
15 |
2 |
17 |
|
Row Percent |
88.23% |
11.76% |
||
|
Count |
B |
9 |
8 |
17 |
|
Row Percent |
52.94% |
47.05% |
||
|
Count |
All Groups |
23 |
10 |
34 |
Table 2 Difference in categorical variables between the groups – fisher’s exact test
It shows, 11.76% of neonates born to mothers in Group A needed resuscitation whereas 47.05 % of neonates born to mothers in Group B required resuscitation. Fisher’s exact test shows a p value < 0.05 which is statistically significant.
Table 1 and Figure 2 show correlation between blood glucose of neonates and mothers – 0.97 (Pearson’s correlation coefficient r) (p<0.05). Comparison of fasting duration in hours for solid and liquid in groups (mean±S.D) was done in Table V, which revealed that in Group A duration of liquid and solid fasting corroborates where fasting guidelines were strictly followed. But in Group B duration of both solid and liquid fasting is prolonged. This indicates that in Group B, due to procedural delay, had prolonged fasting periods (for both solid and liquid), inspite of standard fasting advice.
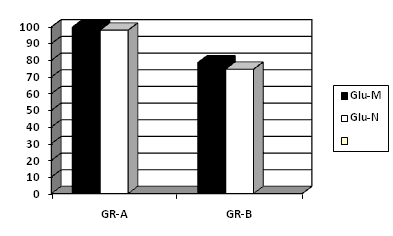
Figure 2 Comparison between blood glucose of infants at birth and mother at delivery by cesarean section. Correlation of maternal blood glucose level with fetal cord blood – 0.97 (pearson’s correlation coefficient r) (p<0.05).
GLU–M: Maternal Mean Blood Glucose Level; GLU–C: Neonatal Blood Glucose Level; GR–A: Group–A, GR–B: Group–B .
Table 1 shows gestational age, birth weight and 1 min Apgar score distribution of infants in the different fasting groups. Analysis reveals that, the difference between Apgar at 1min in Group A and Group B is statistically significant. The difference between need for resuscitation in Group A and Group B is statistically significant.
Figure 3 shows point biserial correlation coefficient. Correlation between glucose levels and need for resuscitation, correlation with maternal glucose level (–0.38) which is significantly negatively correlated (p < 0.05) and with cord blood glucose level (–0.34) (significant at p < 0.05). FIG.IV. shows correlation of newborn blood glucose level compared to that of their mothers at delivery (mg/dl). FIG. IV shows correlation of newborn blood glucose level compared to that of their mothers at delivery (mg/dl).
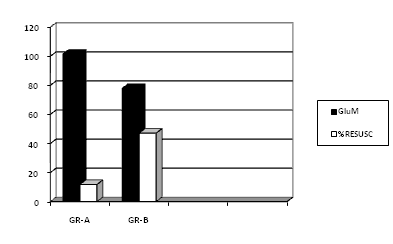
Figure 3 Comparison between Maternal Blood Glucose Levels and Need For Resuscitation (Point Biserial Correlation Coefficient).
With maternal glucose level – 0.38 (significant at p < 0.05).
With cord blood glucose level – 0.34 (significant at p < 0.05).
GluM: Maternal Blood Glucose Level; %RESUSC: % of Neonates Requiring Resuscitation; GR–A: Group–A
GR–B: Group–B
Table 1 shows comparison of Apgar score heart rate, respiratory rate, cord blood glucose level in newborn needed resuscitation between the groups, analysis reveals the difference between the 2 groups in terms of Apgar score at 1 minute, need for resuscitation of newborns, number of neonates with peripheral cyanosis, cord blood glucose level at birth (mg/dl) blood glucose of newborn (mg/dl) needed resuscitation (mean±S.D).
This prospective double blind study revealed that, despite maintaining standard ASA fasting guideline.7 in patients for elective C/S, the duration of fasting time got prolonged (Mean duration of liquid fasting in Gr.A: 2.65hrs; in Gr.B: 11.35hrs). The incidence of patients maintaining recommended duration of fasting is only 23% which was revealed during the selection of study population (Figure 1). This finding is consistent with the study of R. S. Ikonen, E. Hagman and P. Pystynen where they revealed 49% of parturient fasted for 6–12hrs, 38% for 12–18 hrs and 13% found fasted over 18 hrs .1
No definite correlation is established yet related to the duration of fasting at which maternal blood glucose level is responsible for declines in fetal blood glucose level below cut–off point (40mg/dl). In the present study, the decrease in maternal (–.76) and cord blood glucose (–.73) were correlated significantly in opposite direction with duration of fasting (P<0.05) signify that, glucose turnover in fasted, pregnant women is many folds greater compared to demographically matched nonpregnant women (Table 2). Moreover this result was similar to the study of Spellacy, W. N, Suhi, W. C, Bradley, B. & Holsinger, K.K.2 This study also revealed the existence of strong positive correlation between maternal blood glucose level and fetal cord blood glucose level (Table II and III) similar to another study of Phillips, L, Lumley, J, Paterson, P. and Wood, C.11
A low Apgar score on the one–minute test reflects neonatal requirements of medical attention .12 Apgar at 1 and 5 min in this study (Figure 2) showed a positive significant correlation (0.58) which reflects the need for resuscitation. The concept of hypoglycaemia in the newborn has changed in recent years and the classification has been revised .4 Similar to the study of Cutberlet, R L and Cornblalth, this study also showed the development of greatest incidence of hypoglycaemia immediately after birth.4,13,14
Newborns with lower blood glucose levels are at increased risk for brain injury. Increased glucose levels after hypoxia or ischemia were not associated with adverse effects in a recent pediatric series .15–17 Intravenous glucose infusion should be considered as soon as possible after resuscitation, with the goal of avoiding hypoglycemia (Class IIb, LOE C). The present study justifies the above reason (Table 1) as 8 newborns who needed resuscitation in Group B were born with labored breathing with heart rate >100, the incidence of blood glucose level revealed from heel prick capillary test about (57.5±5.928) mg/dl in the prolonged fasting group in comparison to (81.5±9.192) mg/dl in the strictly followed liquid fasting group (Group A). Though they have not crossed the cutoff point of hypoglycemia (<40 mg/dl), but the newborns required resuscitation followed by immediate dextrose infusion. Neonates of starved mothers need resuscitation with serum glucose level <40–45 mg/dl, either with oral feeding (2–3 mL/kg D10 in water) or by intravenous infusion dextrose 8mg/ kg/minute.9
Discomfort during the period of waiting before elective surgery can be reduced if patients are prepared with an isotonic health drink, compared to oral water or overnight fasting. The volume of administered fluids does not appear to have an impact on patient’s residual gastric volume and pH, compared to standard fasting regimen. Therefore, patients may have unlimited volume of water and other clear fluid up to 2hrs before induction of anaesthesia .7
Application of concretions of this study in clinical practice in combating effects of inadvertent procedural delay for elective C/S, is to continue intake of isotonic glucose upto 2–3hrs before surgery for birth of a healthy newborn, as strong positive correlation revealed between solid fasting with maternal blood glucose level, in addition, to maintain maternal and fetal cord blood glucose level which is correlated with need for neonatal resuscitation.
In the decades after the publication of Mendelson’s data, the series of triennial confidential reports on maternal death in the United Kingdom highlighted the initial importance of nothing per oral and policies was rapidly introduced. Maternal fasting leads to more rapid decline in blood glucose and rapid lipolysis, fetus can use ketones derived from the mother as alternative fuels for oxidation in situation of glucose deprivation.
We thank Dr. Avijit Hazra, MD, Associate Professor, Department of Pharmacology, Institute of Post Graduate Medical Education and Research for statistical analysis of this research work, without his help and cooperation this study would not have been possible.
No definite cut of point for the duration of fasting at which the blood glucose level in the mother decline have not been derived the variable are continuously correlated over a period of time. The betahydroxybuterate concentration in maternal and cord blood was not assessed, which is suggested a simple diffusion from mother to fetus to use as alternative fuels for oxidation in situation of glucose deprivation.

©2017 Gupta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.