Journal of
eISSN: 2373-6437


Literature Review Volume 17 Issue 1
1Hospital Militar de Especialidades de la Mujer y Neonatología Medicina Crítica en Obstetricia, Universidad Autónoma del Estado de México, México
10Ginecología y Obstetricia/ Medicina Crítica en Obstetricia, Universidad Autónoma del Estado de México, México
11Cirujano Obstétrico de Alta Complejidad (Hospital Materno Perinatal Mónica Pretelini Sáenz, Universidad Autónoma del Estado de México), México
12Hematología/ Trasplante de Médula Ósea, Universidad Nacional Autónoma de México, México
2Ginecología y Obstetricia/ Ginecología Oncológica, Universidad Nacional Autónoma de México, México
3Ginecología y Obstetricia/ Ginecología Oncológica, Universidad Nacional Autónoma de México, México
4Ginecología y Obstetricia, Universidad Autónoma de Campeche, México
5Hospital Militar de Especialidades de la Mujer y Neonatología, Universidad Autónoma del Estado de México, México
6Ginecología y Obstetricia, Universidad Autónoma de San Luis Potosí, México
7Ginecología y Obstetricia, Universidad Autónoma de Yucatán, México
8Ginecología y Obstetricia/ Endocrinología Ginecológica, Universidad Autónoma de Yucatán, México
9Ginecología y Obstetricia/ Perinatología, Universidad Autónoma de Yucatán, México
Correspondence: Jesser Martin Herrera Salgado, Hospital Militar de Especialidades de la Mujer y Neonatología Medicina Crítica en Obstetricia, Universidad Autónoma del Estado de México, México, Tel +52 1 5581399044
Received: January 20, 2025 | Published: February 5, 2025
Citation: Herrera-Salgado JM, Solis-Loria WA, Lepe-Lemus AK, et al. Damage control surgery in obstetrics: literature review. J Anesth Crit Care Open Access. 2025;17(1):6-12. DOI: 10.15406/jaccoa.2025.17.00612
Damage control surgery in obstetrics is a surgical approach used in emergency situations, involving serious complications or risks to the mother. This technique is commonly used in cases of severe obstetric hemorrhage. The aim is the performance of an initial laparotomy on the hemodynamically unstable patient with the goal of rapidly controlling life-threatening injuries. It should be considered when despite the fact the ligation of important arterial vessels has already been performed, bleeding persists and should be establish coagulopathy, particularly if it is associated with hypothermia, acidosis, hypocalcemia, and requirement of vasopressors. During the initial laparotomy, bleeding should be controlled, and partial or temporary abdominal closure is performed, subsequently, the patient must be admitted to physiologic restoration in the Intensive Care Unit, this is followed by planned re-operation, definitive management of the lesions, and abdominal closure. We conducted an up-to-date literature search and present the most important items related to damage control surgery in obstetric patients.
Keywords: Abdominal compartment syndrome, damage control surgery, obstetrics.
A literature review was carried out with the keywords: Abdominal compartment syndrome, damage control surgery, obstetrics. The search included literature from the most recent 10 years, using the PUB MED databases, 73 articles with the keywords were found, then 42 manuscripts from those that included the items to be treated in the review were selected, considering these were related to the topics to be discussed.
Damage control surgery in obstetrics (DCC) is a surgical approach used in emergency situations, involving serious complications or risks to the mother. The ratio of Extremely Severe Maternal Morbidity calculated in the United Mexican States is 5 per 100 estimated births, with a total of 25,092 cases, Obstetric Hemorrhage represents 19.4%, these cases represent true surgical catastrophes that imply morbidity and serious sequelae for the mother and her environment.1,2 The function of the abdominal cavity is to contain the vital organs such as the digestive, urinary, exocrine, circulatory and parts of the reproductive system.3 The main objective of damage control surgery is to contain injuries or acute bleeding and to allow a subsequent definitive and complete repair in a safer context once the metabolic and coagulation alterations have been corrected.4-7 Over the decades, damage control surgery has undergone a significant evolution, in this paper we present a review of the current literature mainly with a focus on obstetrics, which is intimately related to extremely severe maternal morbidity.
Epidemiology of extremely severe maternal mortality and morbidity in Mexico.
In 2023 the maternal mortality ratio was 26.5 deaths per 100 thousand births. The main causes of death were obstetric hemorrhage (19.4%), hypertensive disease (16.2%), abortion (8.3 %).1 The Extremely Severe Maternal Morbidity ratio was 5 per 100 births. The main conditions of Extremely Severe Maternal Morbidity were Hypertensive disorders (61.9%) and Obstetric hemorrhage (19.4%).2 These primary and secondary clinical conditions can potentially lead to the need for damage control surgery.
Anatomical and physiological aspects of the abdominal cavity
The function of the abdominal cavity is to contain the vital organs such as the digestive, urinary, exocrine, circulatory and parts of the reproductive system. The peritoneum histologically is composed of simple flat epithelial cells. The retroperitoneum contains the adrenal glands, aorta, inferior vena cava, duodenum (second to fourth portions), pancreas (head and body), ureters, ascending and descending colon, kidneys, esophagus (distal portion), and rectum. The abdomen derives from three primary germ layers: the ectoderm, which forms the epidermis, the somatic mesoderm, and the splanchnic, which form the skeletal muscle of the abdominal wall, and the intestinal smooth muscle, respectively and the endoderm that makes up most of the digestive tract.3
Damage control surgery
In trauma, damage control surgery (DCC) refers to the performance of an initial laparotomy on the hemodynamically unstable patient with the goal of rapidly controlling life-threatening injuries. It originated with therapeutic packaging to control bleeding from liver injury in the early 1970s.4 Moore et al observed that 82% of deaths after liver trauma were due to hemorrhage secondary to coagulopathy, hypothermia, and acidosis. In 1981, Feliciano et al reported the benefits of abdominal packing. The term "damage control surgery" was first described by Rotondo et al in 1993.5 Harvin et al in their study conducted in Texas from 2016 to 2019 found that no increased risk of complications was demonstrated when performing damage control surgery versus definitive surgery.6 Delaying closure facilitates eventual abdominal re-exploration and reduces the risk of developing abdominal compartment syndrome. Recognized methods of temporary abdominal closure include topical negative pressure (TNP) or vacuum-assisted closure, The Wittman patch, temporal mesh, fascial tension methods (such as fascial traction sutures or sequential mesh tension), Bogota bag, and skin tension.7 In trauma, the rapid/primary surgery stage, is intended to control bleeding and contamination. After bleeding is controlled, the abdominal viscera are examined in detail.8 In critically ill people with a surgical diagnostic, performing an unnecessary procedure can trigger an even greater immune response and lead to intra-abdominal hypertension which can trigger further organ dysfunction.9 Kilic et al., comparing open abdomen management with and without a suction system, found that the use of an active suction system (VAC) is superior to not using it.10 Roberts et al conducted a systematic review, the findings of this review support that CCD should be used with restriction due to uncertainty regarding its effectiveness.11 A study conducted in South Africa found that 1 in 14 peripartum hysterectomies required DCC. Despite the limited data available, obstetric patients who require damage control surgery should be treated according to the same principles as non-obstetrics. In obstetric patients who have experienced massive hemorrhage, damage control surgery should be considered when, despite the fact that ligation of important arterial vessels has already been performed, bleeding persists, in this scenario it should be considered established coagulopathy, particularly if it is associated with hypothermia, acidosis, and the requirement of vasopressors.12
In the context of patients with preeclampsia, although the most common complication of HELLP syndrome is acute kidney injury, surgical complications are outweighed by the high rate of maternal and fetal death. Spontaneous hematoma or hepatic rupture and hepatic ischemia may complicate 0.9-2% of women with HELLP syndrome.13 Severe trauma with massive hemorrhage regardless of its etiology and site of bleeding can lead to acidosis, coagulopathy with hypocalcemia, and hypothermia. Classically DCS has been described in 3 stages. During the initial laparotomy, bleeding and abdominal contamination should be controlled, and temporary abdominal closure (Stage I) is performed. Subsequently, the patient is admitted to Stage II: Physiologic Restoration in the ICU. This is followed by planned re-operation, definitive management of the lesions, and abdominal closure (Stage III). These can also be described in more stages, encompassing the rehabilitation of the patient after discharge from the hospital (see Figure 1).14 Jiménez et al published the results of a retrospective study conducted at a hospital in Guadalajara, Mexico. Uterine rupture, sepsis, and pulmonary thromboembolism were found to be the factors associated with higher mortality in patients undergoing damage control surgery.15 Pereira et al, in 2018, published the results with the use of a temporary closure technique in patients treated at a hospital in Medellín, Colombia.16 Endovascular Resuscitation Balloon Occlusion of the Aorta (REBOA) is a technique of great interest among trauma surgeons worldwide, but the use of this surgical maneuver has not achieved clear results.17 In 2016, Briones et al documented a clinical case in a patient with placental accretion in which a hysterectomy cesarean section with ligation of uterine and hypogastric arteries was performed, however, due to the refractoriness of the bleeding, clamping of the aorta artery was performed for 43 minutes, with favorable results for the patient.18
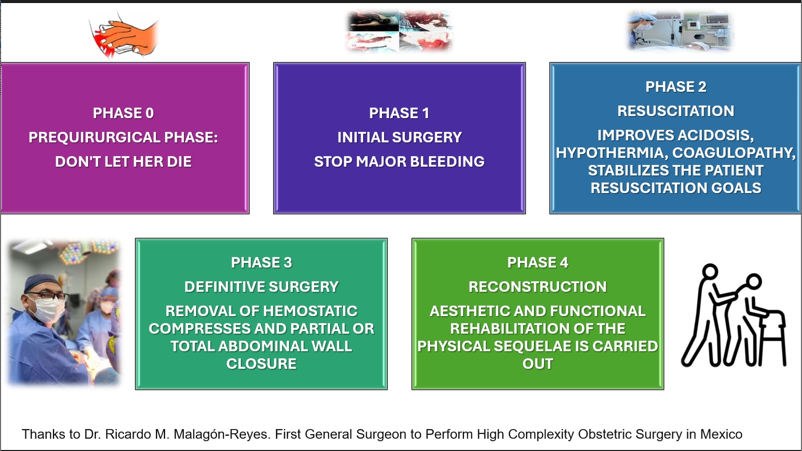
Figure 1 Phases of Damage Control Surgery.
Explains the ideal phases to be followed in the event of a surgical disaster, with the aim of doing so in an orderly manner and with a multidisciplinary team (originally designed by Dr. Jesser M. Herrera-Salgado).
Saskya et al in 2021, through a consensus method based on electronic surveys, content experts agreed on a set of basic outcomes to be pursued for damage control laparotomy, in which it is basically recommended to conduct future trials in clinical research in this regard.19 In 2013, Malagón-Reyes et al published the results of the use of the device called "Bolsa MALA" (MALA Bag), which stands for Greater Abdominal Fluid Absorption, used in DCC in Obstetrics.20 As discussed, the variable underlying content and lack of original research related to indications for CCD suggest that there is substantial uncertainty around when the procedure is indicated and highlights the need to establish evidence-based consensus indications.21 Correct patient selection is crucial to maximizing the benefit of a damage control strategy. Failure to apply the strategy to critically ill patients who merit it will increase their risk of death, although its excessive use will expose patients to the risks of multiple operations, and therefore to a prolonged stay in intensive care.22 In obstetrics, damage control surgery is mainly used in the treatment of patients with bleeding refractory to other measures. In a case series conducted in Cali in Colombia, the outcome of 108 patients requiring CCD was documented. Bleeding control was achieved in all patients, 60% in the first surgical intervention, with a mortality rate of 6.48%. In Mexico City, a series of cases with damage control surgery were recorded due to obstetric hemorrhage, and definitive treatment was achieved in the second surgery in 81% of cases.23
In the clinical practice guideline for Postpartum Hemorrhage (PPH) of the Ministry of Health of the United Mexican States, updated in 2021, it is suggested that abdominopelvic packing (Mikulicz type) be used, that the patient with abdominopelvic packing be admitted to an intensive care unit to continue damage control resuscitation. The suggested recommended abdominopelvic packing time is 48 to 72 hours and abdominopelvic unpacking once the patient's conditions (coagulopathy, acidosis, hypothermia, etc.) have stabilized and corrected.23 In obstetric sepsis, according to some reports, damage control surgery improves the survival of this type of patient, however, this information is limited to a few case reports.24 Among the causes that the surgeon may face, there are cases in which the pelvic abdominal damage is secondary to fractures. After bleeding control has been achieved, an endoscopic examination should be performed to check for evidence of rectal, vaginal, genitourinary, or anal lesions to determine the need for further studies.25
Abdominal compartment syndrome
Intra-abdominal hypertension (IAH) is associated with significant morbidity and mortality. Abdominal compartment syndrome (ACS) represents the end of a pathophysiological spectrum that begins with normal intra-abdominal pressure (IAP) and continues through worsening degrees of HIA. According to the 2020 Journal of Gastroenterology of Mexico, abdominal compartment syndrome is defined when two or more anatomical compartments have an intra-abdominal pressure with a sustained value > 20 mmHg, associated with organ failure. The frequency is 2% and up to 36.4%.26 The main risk factors for the development of ACS are divided into those that condition a decrease in the compliance of the abdominal wall (acute respiratory failure, abdominal surgery with primary or tension closure, abdominal wall hematoma, severe abdominal trauma, severe burns with abdominal retraction, obesity), Intra-abdominal volume increase (gastroparesis, gastric distention, ileus, volvulus, colonic pseudo-obstruction, intra-abdominal tumor, tumors or abscesses, hemoperitoneum, pneumoperitoneum, ascites) factors that increase capillary leakage (sepsis and septic shock, complicated intra-abdominal infection, aggressive administration of fluids, polytransfusion, extensive burns, severe polytrauma, acidosis, hypothermia, and coagulopathy).27
Clinical examination is insufficient to detect elevated IAP. The technique or gold standard for diagnosis is abdominal pressure measurement with a bladder access catheter. It is recommended that patients be supine with relaxed abdominal musculature in the final expiratory phase of breathing and the transducer zeroed in the iliac crest at the level of the mid-axillary line on the longitudinal axis. The gold standard for intermittent IAP measurements is through the use of a bladder transducer with a maximum instillation volume of 25 mL of sterile saline. IAP should be expressed in mmHg and measured at the end of expiratory in the supine position after ensuring that abdominal muscle contractions are absent and with the transducer set to zero. IAP is about 5 to 7 mm Hg in critically ill adults. IAH is defined by a sustained or repeated pathologic elevation of IAP greater than 12 mm Hg. ACS is defined as a sustained IAP of more than 20 mm Hg (with or without an abdominal perfusion pressure PPA less than 60 mm Hg) that is associated with one or more organ dysfunctions, often renal.28
Intra-abdominal hypertension is classified as grade I if IAP is 12–15 mmHg, grade II with IAP 16–20 mmHg, grade III with IAP 21–25 mmHg, and grade IV IAP >25 mmHg. Mortality is 26.1% for grade III abdominal compartment syndrome, while it is 100% for grade IV. ACS mortality remains high (50%-60%) even when decompression of the abdomen is performed early, hence the importance of detection and treatment of AIH before end-stage organ damage occurs.29 The use of “Bolsa de Bogotá” is one of the cheapest and most efficient ways to surgically treat patients with ACS. Colombian surgeon Oswaldo Borraez was the first to use this technique. The Bogota pouch acts as an airtight barrier that prevents evisceration and fluid loss, and the abdominal contents can be visually inspected, which is particularly useful in cases of ischemic bowel. Dhoj et al documented results of the application of Bogotá Bag during abdominal closure for patients diagnosed with ACS or prone to developing ACS, and this was a feasible technique with minimal procedure-related morbidities.29 Muhammad et al at Mayo Hospital Lahore, Pakistan, concluded that the use of this device is an effective and useful means of open abdominal wound closure to prevent complications due to viscera exposure, i.e., evisceration, intestinal injury, fluid loss, temperature, and complications secondary to the tension of definitive wound closure.30 Samimian et al concluded that elevation of the head angle from 0 to 30 degrees significantly increases IAP, which may increase the frequency of IAH and ACS with its statistical bias.31 According to experts, the measurement of bladder intra-abdominal pressure remains the gold standard for the determination of IAP and should be monitored at least every 6 hours. In this study, Smereczyński et al performed the following steps with POCUS; 1st stage evaluate the status of gastric contents, correct placement of the gastric tube, intestines, at 6-hour intervals, then echocardiography, blood flow in the hepatic artery, renal arteries and portal system in the initial stage of follow-up, 2nd stage evaluation of intraluminal content and intestinal motor function. The 3rd stage consisted of monitoring the occurrence of ascites.32
Nonsurgical therapeutic options for the treatment of intra-abdominal hypertension include control of excessive muscle contraction, evacuation of luminal contents by decompression, evacuation of abdominal fluid by drainage, and guided volume resuscitation. After surgical laparotomy for compartment syndrome, the abdominal fascia may be left unclosed. With less strain on the abdomen, diaphragmatic excursion can increase, leading to better ventilation and reduced peak airway pressures. Compression of the inferior vena cava and circulatory system are resolved, leading to improved cardiac output and the ability to disconnect patients from vasopressor support. Acute kidney injury is reversed with less compression on the renal vessels and ureters.33 Li et al. reported that they had lower mortality rates with patients managed with open abdomen compared to the abdomen group with initial definitive closure (ACD), as well as lower hospital costs.34
In summary, abdominal compartment syndrome causes multi-organ dysfunction that leads to varying degrees of death in the patient without effective treatment or correction of the etiologies involved (see Figure 2).

Figure 2 Compartment Abdominal Syndrome.
Explains the key points of the multi-organ impact caused by abdominal compartment syndrome (originally designed by Dr. Ana E. Montes-García).
Damage control in obstetric hemorrhage
Postpartum hemorrhage is a significant contributor to maternal morbidity and mortality. It has been defined as an estimated blood loss greater than 500 ml vaginally or greater than 1000 ml by caesarean section. In 2017 the American College of Obstetricians and Gynecologists (ACOG) defined it as a cumulative blood loss greater than 1000 ml with signs and symptoms of hypovolemia within 24 hours of the birth process, regardless of the route of birth. Primary hemorrhage is defined as hemorrhage that occurs in the first 24 hours after delivery and secondary hemorrhage occurs 24 hours to 12 weeks after delivery.35 Patients who are bleeding during labor, have a history of PPH, anemia with hematocrit less than 30%, hemorrhagic diathesis or known coagulopathies, and abnormally inserted placenta are at high risk for PPH.36 Hemostatic resuscitation (HR) should include physiological correction goals and include a surgical approach with DCC in its different phases. HR describes a systematic approach to minimize bleeding, prevent acidosis, hypothermia, hypocalcemia, and coagulopathy, as well as maximize tissue oxygenation with the support of the Intensive Care Unit (ICU). The WOMAN trial studied the effect of early administration of TXA on maternal mortality after hysterectomy and other surgical morbidities in patients with PPH. Death from hemorrhage was significantly reduced in patients who received TXA. Rotational thromboelastometry (ROTEM) and thromboelastography (TEG) are used to perform rapid viscoelastic analysis of the kinetics of all stages of clot formation, reduce costs, and allow efficient use of blood components in patients with severe postpartum hemorrhage.37 Bienstock et al. suggest the following recommendations in massive hemorrhage; transfusion of red blood cells, PFCs, and platelets in a 1:1:1 ratio even consider uterine artery embolization with interventional radiology.38
There is substantial variation in PPH management guidelines among the 4 major obstetrics organizations: American College of Obstetrician and Gynecologists practice bulletin (ACOG), Royal Australian and New Zealand College of Obstetricians and Gynecologists (RANZCOG), Royal College of Obstetricians and Gynecologists (RCOG), and Society of Obstetricians and Gynecologists of Canada (SOGC) highlighting the need for better and more consistent evidence.39 It is considered most importance to establish standardized measures for the resuscitation of patients with massive bleeding of obstetric origin. These standards should be approach in order to practice the safest and most effective interventions for the patient.40
In pregnancy, IAP behaves mildly different from the non-pregnant patient, before 20 weeks (5-7 mm Hg), 20-26 weeks (8-10 mmHg), 26-32 weeks (11-12 mmHg), after 32 weeks (11-12 mmHg) and postpartum (8-10 mmHg). IAH in pregnancy is defined as a sustained elevation of IAP ≥14 mm Hg, ACS as sustained IAP >25 mm Hg that is associated with new organ failure/dysfunction.41 Some recommendations are established based on the physiological considerations mentioned above:
Chronically elevated IAP during pregnancy becomes pathological when acute changes exceed the capacity of the abdominal cavity to compensate for these increases in content. The incidence of AIH is approximately 6%, compared to an incidence of IAH of 25% and an incidence of ACS of 5% in mixed ICU populations. Case reports describe complications of preeclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, decreased platelets), disseminated intravascular coagulation, and massive transfusion that convert compensated AIH into ACS. Conditions such as appendicitis, cholecystitis, pancreatitis, and trauma are among the most common non-obstetric problems that can occur in pregnancy and lead to these complications (41). Rare conditions such as hepatic rupture secondary to preeclampsia are lethal even doing DCS in expert hands and specialized obstetrics intensive care units (42).
In 2023, the calculated maternal mortality ratio was 26.5 deaths per 100 thousand births. The main causes of death were obstetric hemorrhage (19.4%), hypertensive disease (16.2%), abortion (8.3 %), therefore, improving surgical management strategies, including DCC in obstetric hemorrhage, represent a priority.1 Several authors such as Chabot et al. (2017), Benz et al. (2017), Harvin et al. (2017), Sharrock et al. (2016), Kanat et al. (2016) describe the indications for open abdominal management, in these studies the indications were mainly cases of trauma from traffic accidents and gunshot wounds,4-8 we can assert that in cases of obstetric hemorrhage the mechanism of damage and the physiological response to the injury are similar, thus, damage control should follow the principles of a trauma patient as suggested by Pacheco et al. (2018).12 In studies conducted by Coccolini et al. (2018), Kılıç et al. (2018), Roberts et al. (2015) found overweight body mass index in most of their patients, which presents similarity in terms of the morphological biotype presented by obstetric patients.9-11 In Mexico, in studies conducted by Pacheco et al. (2018), as well as Jiménez et al. (2020), multiparity was a predominant finding,12,15 in studies conducted internationally, Messina et al. (2021) reported a case of hemorrhage of obstetric cause complicated with hepatic rupture with a history of multiparity in the same way,13 Karamarcovic et al. documented similar antecedents.14 The difficult control of bleeding has been a very frequent complication that has led to novel strategies that have been carried out heroically without known pathophysiological history with only a few case reports, such as those reported by Briones et al. (2016) in this case clamping of the aorta was done temporarily to prevent the death of a patient with placental accretion and massive bleeding saving the patient's life,18 Ordoñez et al. (2020) also documented a case of aortic clamping in a case of massive abdominal trauma with retroperitoneal hematoma with equally successful results.17 Most authors such as Byerly et al. (2022), Roberts et al. (2015), Matsumoto et al. (2021), Simbanassi et al (2014), Montalvo et al. (2020), report the need for multiple interventions in cases of damage control surgery, due to new bleeding sites, uncontrolled bleeding, collection or abscess formation, and complications of wall closure.19,21,22,24,25,26 In studies reviewed we found that the mean days of hospitalization were greater than 8 days. Authors such as Sánchez et al. (2013), Roberts et al. (2016), Joshi et al. (2017), however, were patients who were in the intermediate care ward and not in intensive care.27-29 Muhamad et al. (2016) and Samimian et al. (2021) recorded cases of patients with abdominal compartment syndrome and intestinal complications that required chronic stay in intensive care, and it was even necessary to leave the abdomen without definitive closure even at discharge from hospitalization.30,31 Findings in the study published by Malagón et al (2013) described a technique initially in patients with the need for open abdominal management for various causes, the most frequent being hemorrhage and obstetric sepsis.20
Hemorrhage as the main cause is described in most international publications, in the publications of Giouleka et al. (2022), Wormer et al. (2022), Carvajal et al. (2020), Bienstock et al. (2021), Dahlke et al. (2015), Ortega et al. (2018), in these documents the predominant etiology is uterine atony as the most important cause of bleeding, Worldwide, similarly, massive bleeding with higher morbidity and mortality is secondary to placenta accreta spectrum procedures.35-40
In a case series carried out in Cali, Colombia, the outcome of 108 patients who required DCC with described complications was documented, such as surgical site infections (28.7%), intra-abdominal abscess (20.3%), evisceration (10.1%) and abdominal compartment syndrome (9.26%), with hypovolemic shock and coagulopathy as the most important cause of death.23 Similarly, in Mexico City, a series of cases with damage control surgery for obstetric hemorrhage was recorded, whose complications were infectious (31%) and disseminated intravascular coagulation (19%). IAH is defined by a sustained or repeated pathologic elevation of IAP greater than 12 mm Hg. ACS is defined as a sustained IAP greater than 20 mm Hg that is associated with new organ dysfunction, often renal.28 Intra-abdominal hypertension and abdominal compartment syndrome occur with high prevalence in intensive care unit patients and cause significant mortality in obstetrics and non-obstetrics patients. Mortality is 26.1% for abdominal compartment syndrome and grade III, while it is 100% for grade IV.29 As a pathophysiological principle, in the context of abdominal trauma, the amount of intraperitoneal fluid will be increased and, if there is no adequate drainage, it tends to accumulate with the appearance of subsequent complications, much more pronounced in the obstetric patient, given the changes in total body water and oncotic pressure.
Damage control surgery is an emergency surgical procedure that aims to contain the bleeding and/or contamination phases of a surgical catastrophe. It is generally indicated when there is severe metabolic acidosis, coagulopathy, severe hypothermia, hypocalcemia, associated with a difficult control of bleeding that endangers the patient's life. This procedure aims to contain bleeding mechanically in an orderly manner, while correcting coagulation disorders, acidosis, hypothermia, electrolyte alterations, mainly hypocalcemia in the context of coagulopathy, in addition to improving the state of shock caused by the trauma or hemorrhagic/septic event that triggered it. In a later surgical time, once the initial pathophysiological complications have been corrected (this in a period of 48 to 72 hours generally) the definitive surgery should be performed. In the critically ill obstetric patient, the same objectives and pathophysiological principles should be considered, taking into account some changes related to her condition (mainly slightly higher intra-abdominal pressure and procoagulant status), the resuscitation goals are similar except for the fibrinogen levels (200 mg/dL). The most important cause of damage control surgery in Obstetrics is hemorrhage, a situation that every obstetrician must be trained to attend, this situation is generally faced unexpectedly, so it is essential to train continuously and with simulations this complication. We suggest that each health unit has a specialized team to attend catastrophic surgeries, in our opinion, it should be made up of the Obstetrics - Gynecology and General Surgery service with training in the medical and surgical treatment of this type of complications.
None.
None.

©2025 Herrera-Salgado, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.