Journal of
eISSN: 2373-6437


Research Article Volume 6 Issue 3
1Department of Anesthesiology, Santiago de León Clinic of Caracas, Venezuela
2Department of Anesthesiology, Pontificia Universidad Católica de Chile, Venezuela
3Department of Clinical Anesthesiology Ávila, Venezuela
Correspondence: Carolina Frederico Avendaño, El Cafetal Urbanization, Santa Clara Sector, El Callao Street Quinta Campo Blanco, postal code 1061, Caracas-Venezuela, Tel +584142567161
Received: December 13, 2015 | Published: November 30, 2016
Citation: Frederico AC, CortÃnez LI, RamÃrez-Paesano CR (2016) Comparison of Model and Schnider CortÃnez Infusion of Propofol Target Controlled 3 Mcg/Ml, to Biophase in Healthy Volunteers. J Anesth Crit Care Open Access 6(3): 00230. DOI: DOI: 10.15406/jaccoa.2016.06.00230
Objective: To compare the clinical and Cortínez Schnider models with "TCI" systems for infusion of propofol effect-site concentration of 3mcg/ml in healthy volunteers.
Methods: 10 healthy volunteers were prospectively studied in 2 occasions. Propofol was administered with Cortínez or Schnider model as randomized. Times and concentrations of propofol achieved at the time of loss and recovery of consciousness (LOC and ROC), mass of drug administered, BIS values and hemodynamic variables were compared. Statistical analysis was with paired Wilcoxon. A p≤0.05 was considered significant.
Results: The initial bolus of propofol was higher (1.4 (1.3-1.6)mg/kg versus 0.9 (0.7-1.3)mg/kg, p=0.005) and LOC occurred before (1.33 (0.67 to 6.83) versus 3.87 min (1.66 to 11.08)min, p=0.02) with Cortínez model compared to the model of Schnider. The model predicts a concentration Cortínez biophase the LOC of 2.6 (1.65 to 3.0) mcg/ml. With the LOC occurs Schnider 3.4 (0.2 to 10.3) min after reaching the target of 3 mcg/ml. (P=0.001). BIS values, infusion rates and hemodynamic variables were similar with both models after 20 minutes infusion (P>0.5). Recovery (ROC) was longer with the model Cortínez 11.6 (8.1 to 16.2) min vs 8.5 (4.7 to 15.5) min. (P=0.003).
Conclusion: The Cortínez model is a good alternative to the Schnider model for use in effect-site TCI mode in normal weight subjects. With the target used in this study (3mcg/ml), the slower Ke0 incorporated in the Cortínez model better discriminated the moment of LOC.
Keywords:propofol, pharmacokinetics, target controlled infusion, Ke0
The administration of propofol by TCI infusion systems (target controlled infusion) has advantages over manual management, allowing a more precise dosing and easily, according to a desired concentration using pharmacokinetic models (TCI plasma) or pharmacokinetic-pharmacodynamic models (TCI biophase) incorporated in the pumps. These models take into account the influence of different variables of each patient (weight, height, age, sex, body mass index (BMI), lean mass index (IMM)) in the dosage of the drug. There are, however, multiple models for infusion of propofol, with characteristics very distintas,1 this diversity has generated confusion among clinicians when choosing the best model available to their patients anesthesiologists. The incorporation of models to represent the best population diversity is a real need to simplify the current clinical stage of the TCI.
Recently, Cortínez et al.2 have developed a pharmacokinetic model of propofol from data of normal-weight and obese patients from 3 studies.3,4 The model uses a Allometric Cortínez total body weight scale to characterize the changes in volumes and clearances of propofol to increase the size corporal.2 This scale adjusts better, the nonlinear changes of clearance of propofol to the weight, observed in patients with a wide range of weight corporal.5 In a recent study Cortínez et al.6 characterized the temporal profile of the measured effect of propofol with BIS in obese population, finding a temporary profile (time to peak effect 2.1min) similar to That Previously Described in non-obese patients With monitor.7 To date, the model adjusted for use Cortínez EVALUATED biofase6 no has-been in People with regular weight.
Schnider3 model is a pharmacokinetic-pharmacodynamic has been widely studied and validated in non-obese population model. This model allows dosing to biophase it incorporates a constant that describes the rate of equilibration between plasma and brain (ke0) derived from a canonical electroencephalogram analysis. This constant however, it has been questioned in the literature as described speeds faster than those found in other studies8,9 balance, questioning the predictive ability of the model in the first minutes of anesthetic induction.
The aim of our study was to clinically evaluate the pharmacokinetic model-farmacodinámicode Cortínez6 by perfusion systems with TCI goal biophase in non-obese population. For this we propose to perform a double-blind crossover study in healthy volunteers comparing this model with the model of Schnider. Our hypothesis is that Cortínez model should show good performance in TCI biophase in non-obese population due to its pharmacokinetics allometrically climbing weight, patients from a wide range of body weight, and its derivative Ke0 BIS.
This study was registered with ClinicalTrials.gov (Identifier: NCT02155517), in May 2014, by Carolina Frederico A MD After the approval of the Institutional Bioethics Committee and after obtaining written informed consent, 10 healthy volunteers, aged between 18 and 60 years and body mass index (BMI) <30kg/m2, were recruited ≥ II patients with ASA physical status, history of alcohol abuse, snuff and drugs, chronic use of any type of anti-anxiety medication and cardiovascular field, BMI <18 or>30kg/m2, neurological or psychiatric disorders and pregnancy were excluded. It prior to each day of the study, volunteers were asked to abstain from alcohol and any kind of medication, as well as keep fasting for 8 hours.
A controlled clinical trial, double blind, crossover two days separated by a time interval of 7 days after the initial day study was designed. On the first day of study, volunteers were randomized into two groups (Group S: Schnider, Group C: Cortínez), using computerized random number table (Analysis SPSS version 18, IBM Corp., Armonk, NY), for infusion of propofol with one of the two models to evaluate. Alternative pharmacokinetic model was used on the second day of the study. The volunteers were blind to assignments.
Prior to administration of propofol volunteers they received 5L/min of supplemental oxygen through a face mask. 20G venous access was installed in the back of his right hand, and 500ml of saline solution was administered. The connection of propofol was installed as possible to the proximal vein to decrease the dead space. Propofol was administered for 20minutes with the TCI Syramed® μSP6000 Premium (Arcomedag, Medical System, Switzerland) system, with a fixed goal in biophase 3mcg/ml. The data is automatically recorded in a computer laptop, every 5 seconds for later analysis with the InterLink program (Arcomedag, Medical System, Switzerland).
Before anesthetic induction volunteers were monitored with electrocardiogram, pulse oximetry, noninvasive blood pressure (NIBP). They also monitored Cardiac Output (CO) non-invasively measured by electrical velocimetry based on transthoracic impedance differential, using the Monitor ICON® (Ospyka Medical Inc., California, USA) and Bispectral Index (BIS VISTA™ Aspect Medical Systems Newton USA), through a Quatro (Covidien, MA USA) sensor applied to the forehead of the patient according to manufacturer's recommendations. BIS data is recorded every 5 seconds on the computer via the Window Hyperterminal program.
After obtaining a stable signal A (index higher signal quality of 90% with a lower impedance 5k), and monitor ICON, the anesthesiologist responsible administered 1 mg/kg of lidocaine 2% to a minute before administration of propofol, to reduce pain. After the start of the infusion time loss of consciousness (LOC) it was determined. LOC was defined as the transition from level 3 to 2 on the modified scale of consciousness and sedation assessed by the observer (OAA/S), repeating the name of the volunteer every ten seconds.
Upon completion of the infusion time (20minutes), the target to 0mcg/ml in the TCI device is set, and from that moment began measuring the recovery time of consciousness (ROC), defined as the response to the call by name every 10 seconds. Timekeeping LOC and ROC was in charge of a second anesthesiologist blind to the pharmacokinetic model. The study ended once the time was registered ROC.
The sample size was calculated to find a mean difference between variables other measures 20% (90% power, 5% alpha error), considering a variability of 20%. To compare the variables measured with both models, the Wilcoxon test was applied for paired samples. For statistical analysis software SAS Institute Inc. Values are expressed as median and range were used.
10 healthy volunteers (6 males, 4 females), median age 30 years (19-48 years), weight 63.5kg (52-95kg), Height 169.5cm (163-195cm), BMI were studied 22.7kg/m2 (18.4 to 29.3kg/m2). All volunteers completed the study in two periods according to the protocol. No complications of any kind were reported. All volunteers lost consciousness with the target assigned regardless of the model used. With Schnider, the time to reach equilibrium was 1.5 minutes (1.25 to 1.58 minutes) and LOC occurred on average 3.87 minutes (1.66 to 11.8 minutes), after reaching the target 3 mcg/ml. (P=0.0019). With Cortínez, the time to reach equilibrium was 1.9 minutes (1.75 to 2.08 minutes) LOC being observed at 1.33 minute (1.66 to 11.8 minutes), (p=0.35). Table 1 compares the relevant clinical data observed during induction of anesthesia with propofol receiving Schnider or Cortínez respectively. Figure 1 shows the time profile of each patient BIS both models. During the maintenance phase of anesthesia, BIS values with both models continued to decline despite holding the fixed target in 3mcg/ml (Figure 2 & 3). We found no differences in the values of A (43 [31-59] vs 41 [26-54]; p=0.11), or the infusion of propofol (8.0mg/kg/h vs 8 0mg/kg/h; p=0.84) at the end of the holding period using the model of Schnider or Cortínez respectively. The total mass of propofol delivered throughout the study period was 255.0 mg (220.0 to 284.0mg) with Schnider and 265.5mg (220.0 to 373.0 mg) with Cortínez (p = 0.041). After discontinuation of propofol, recovery time of consciousness was 8.45 minutes (4.67 to 15.5 minutes) with the model of Schnider and 11.62 minutes (8.08 to 16.17 minutes) with Cortínez (p=0.003). BIS values when the ROC were similar (Schnider: 71 [66-77] vs Cortínez: 73 [67-78]; p=0.24). The EC reached when the ROC were also similar (Schnider 0.96 mcg/ml [0.6 to 1.43] vs Cortínez 1.05mcg/ml [0.76 to 1.42]; P=0.54). No differences in baseline hemodynamic variables, the LOC, at 20min, and the ROC between the two groups (all p>0.05). Measured hemodynamic changes are shown in Figures 4 & 5.

Figure 2 Trend BIS at the time of the 10 volunteers with both models PKPD (Schnider and Cortínez). The thick lines represent the median range for each group.

Figure 3 Box plot showing the median interquartile range of the time course of the BIS of 10 volunteers with both models PKPD.
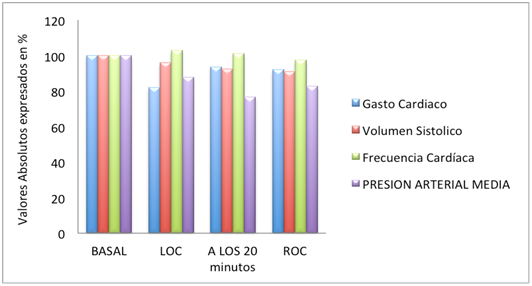
Figure 4 Absolute values of hemodynamic changes expressed in percentage (%) with the model of Schnider.
(N = 10) |
|||
Model |
Model |
||
Schnider |
Cortinez |
P |
|
Basal BIS (BIS Units) |
97 (96-97) |
97 (90-97) |
0.55 |
PAM baseline (mmHg) |
83 (78-102) |
80 (70-95) |
0.06 |
Basal GC |
5 (4.3-6.9) |
5.3 (3.5-6.2) |
0.19 |
Basal heart rate (bpm) |
65 (51-88) |
62 (55-81) |
0.57 |
Initial bolus of propofol (mg/kg) |
0.9 (0.7-1.3) |
1.4 (1.3-1.6) |
0,005 * |
LOC time (min) |
3.87 (1.66-11.08) |
1.33 (0.67-6.83) |
0.02* |
Total dose of propofol when LOC (mg/kg) |
1.4 (0.7-2.5) |
1.4 (1.3-2.4) |
0.95 |
Ce the LOC (mcg/ml) |
3.0 (3.0-3.0) |
2.6 (1.65-3.0) |
0.02* |
BIS to LOC (Units BIS) |
63 (44-77) |
56 (29-90) |
0.16 |
PAM to LOC |
76 (64-87) |
69 (62-84) |
0.16 |
GC LOC |
5.1 (4.6-6.7) |
5.3 (4.1-6.9) |
0.91 |
LOC heart rate (bpm) |
69 (54-93) |
64.5 (57-85) |
0.83 |
Table 1 General data during anesthetic induction
Values expressed as median and range *P (<0.05).
In the present study models they were compared Cortínez2 and Schnider3 during TCI of propofol, with target biophase (target 3mcg/ml) in 10 healthy volunteers. Both models generally similar levels of hypnosis (BIS) was obtained without hemodynamic depression during anesthetic maintenance. The biggest difference was observed in the higher bolus delivered by the model Cortínez to start resulting in TCI faster induction without evidence of statistically significant between LOC times and equilibration times to 3mcg/ml delay. Our results show that the model Cortínez is a good alternative to the model of Schnider for use with TCI goal biophase in normal weight subjects.
The size of the initial bolus TCI biophase depends basically the central volume and speed of balance between plasma and effect-site represented by the constant ke0. Whereas the central volumes of both models studied are similar (Table 2), is then ke0 slower, incorporated Cortínez model (average time to peak effect of 2.1min) compared to that of Schnider (time average peak effect 1.6min), responsible for the largest and the smallest concentration bolus biophase (Ce) reached the LOC. From a clinical point of view, the model overestimates Schnider Ce and subdosifica the initial bolus, which is evidenced by the fact that all the subjects lost consciousness several minutes after reaching the target of 3mcg/ml (3.4 (0.2 to 10.3) min), showing that the ke0 of Schnider is inadequate to predict loss of consciousness induction. Slower balance between plasma and brain as used in the model Cortínez bolus resulted in a greater and more consistent Ce predictions with the response observed in our study in this period. Previous studies agree with this result.9–12
|
Schnider |
Cortine |
|
|
V1 (L) |
4.27 |
4.03 |
|
V2 (L) |
27.9 |
26.5 |
|
V3 (L) |
238 |
213 |
|
Cl1 (L/min) |
1.87-1.56 * |
1.77 |
|
Cl2 (L/min) |
1.84 |
1.81 |
|
Cl3 (L/min) |
0.84 |
0.79 |
|
Ke0 (min-1) |
0.456 |
0.19 |
|
Tpeak (min-1) |
1.6 |
2.1 |
Table 2 Parámetros pharmacokinetic Cortine Schnider and representative of our study in 30, 63kg, 170cm Adult. (*Female Male).
Tpeak: Time peak effect in minutes.
During the maintenance phase, after reaching the desired goal, BIS values did not stabilize as expected and continued to fall during the 20 minute infusion with both models (Figure 2 & 3). One possibility to explain this result is that the models studied have been unable to maintain stable concentrations during the study period. Unfortunately not measured plasma levels of propofol in our study to confirm or reject this possibility. Alternatively, the progressive decrease in post-LOC BIS may reflect that most patients require drug mass loss of consciousness for anesthetic maintenance, adrenérgica13 increased activity during the alertness may explain the higher requirements of propofol in the induction compared with maintenance. A similar finding was reported by Coppens et al.8 noting a progressive decline of the BIS for 11 minutes post-LOC in patients receiving propofol with Schnider model, despite maintaining the fixed target concentration. Consistent with this hypothesis, our results show that during the anesthetic recovery the plasma concentrations achieved ROC propofol concentrations are lower than predicted the LOC with both models (Table 3 & Figure 6). It has been seen that the neural mechanisms governing the transition between loss and recovery of consciousness are different.14 Although hypothetical, the concept of neuronal inertia has been used to describe this phenomenon of resistance to change between stable states of consciousness and unconsciousness and may explain the observed results with both models.15,16
N = 10) |
|||
Model |
Model |
||
Schnider |
Cortine |
P |
|
Ce the LOC (mcg/ml) |
3.0 (3.0-3.0) |
2.6 (1.65-3.0) |
0.02 * |
Ce to ROC (mcg/ml) |
0.96 (0.6-1.43) |
1.05 (0.76-142) |
0.24 |
BIS to LOC (Units BIS) |
63 (44-77) |
56 (29-90) |
0.16 |
BIS to ROC (Units BIS) |
71 (66-78) |
73 (77-79) |
0.24 |
The ROC TIME (minutes) |
8.46 (4.67-15.5) |
11.62 (8.08-16.17) |
0,003 * |
Table 3 Data from the valoress predicted concentrations for each model (Ce) and the BIS and ROC tiemposLOC.
Values expressed as median and range.
*P (<0.05)
In the phase of awakening, recovery time of consciousness it was lower compared to Cortínez Schnider (8.46minutes [4.67 to 15.5min] vs 11.62min [8.08 to 16.17min], P 0.003, respectively). The later wake Cortínez observed in our study may be due to the higher residual induction bolus received as maintenance rates after 20 minutes were similar with both models (8.0mg/kg/h). He based pharmacokinetic similarities between the two models in non-obese adults, it is expected that longer periods of perfusion differences in times of awakening should be minimal. A potential advantage of Schnider model regarding Cortínez not evaluated in this study, is the ability to adjust its clearance with respect to sex, which could generate a better adjustment of infusion rates under this covariate in longer infusions.
One aim of our study was to quantify the hemodynamic impact of each model including cardiac output. The fastest induction by bolus greater magnitude observed with Cortínez model did not generate significant hemodynamic changes. The differences in the values of stroke volume did not exceed 10% decrease from baseline in both models, so we can say that the target used (3mcg/ml), both models provide adequate hemodynamic stability study population. The observed differences between the two models in terms of higher initial bolus Cortínez model should be considered when defining the target plasma concentration, especially when it comes to vulnerable populations where hemodynamic consequences could be of greater impact.
The proper choice of the model of propofol in TCI for a specific patient may be confused by the large number of existing models. The main advantage Cortínez model is its allometric structure that allows patients to better adapt to a wide range of body weight. Other models subsequent propofol have corroborated the validity of the dosing alometría propofol corporal17,18 patients of different weights. It is desirable that these new models may be available in updated versions of the TCI systems to facilitate and improve the technical limitations observed with the current models versions.
Cortínez model is a good alternative to the model of Schnider for use with TCI goal biophase in normal weight subjects. With the lens used with the Ce predicted slower Ke0 built with Cortínez model allows to better discriminate the time of the LOC.
None.
The authors declare there are no conflicts of interest.
None.

©2016 Frederico, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.