Journal of
eISSN: 2373-6437


The anterior and superior mediastinal masses lie in close proximity with the airway, major vessels, heart and can cause compression of the airway and obstruction of the major vessels leading to reduced cardiac output. Induction of general anaesthesia, handling of the airway and tumour manipulation intraoperatively can cause life-threatening haemodynamic changes. We present the successful management of an infant with anterior mediastinal mass in impending cardiorespiratory collapse that underwent tumour resection and discuss the anaesthetic challenges encountered. Careful decision making, availability of equipment and expertise needed to manage such complicated cases and also a team work with good communication between the members is essential for a favourable outcome.
Keywords: anesthesia, infant, mediastinal neoplasm, shock
Anterior mediastinal mass in a child has serious life threatening anaesthetic implications and its management is a challenge for the anaesthesiologist. The anterior and superior mediastinal masses lie in close proximity with the airway, major vessels and heart, and can cause compression of these structures leading to reduced cardiac output. Sedative premedication, induction of general anaesthesia (GA) with or without skeletal muscle relaxants, intermittent positive pressure ventilation (IPPV), change of posture, and tumour manipulation or resection can cause catastrophic airway and cardiovascular collapse even in a previously asymptomatic child.1−4 Induction of GA may cause airway and cardiovascular collapse even in a previously asymptomatic child. In the presence of severe symptoms such as positional dyspnoea, orthopnoea, stridor, syncope and superior vena cava syndrome administration of GA may be fatal.5,6 Airway collapse may occur even after endotracheal intubation and an emergency tracheostomy performed to relieve the obstruction may prove futile, as the obstruction may be distal to the tube.7 We present the successful management of an infant with mediastinal tumour in circulatory shock, the difficulties faced in decision making and discuss the anaesthetic challenges encountered.
A 5-month-old girl (weight=5.5kg) was admitted with a history of runny nose, noisy breathing and cough for 15-days. A chest radiograph showed homogeneous opacity occupying the right hemithorax. Computed-tomography (Figure 1A) showed an anterior mediastinal mass compressing the right bronchus and cardia, near-total collapse of right lung, encasement with compression of superior vena cava, brachiocephalic veins and aorta, and loss of fat-plane between the lesion and pericardium. A day prior to surgery, she was febrile, heart rate (HR)=180 b/min, blood pressure (BP)=82/46 mmHg, respiratory rate (RR)=50 breaths/min, oxygen saturation=99% (on oxygen 2 L/min via nasal-prongs) and reduced air entry in right mammary area. Haemoglobin was 12.9 g%, platelets=469000/mm3, leucocyte count=18280/mm3, sodium=141 mEq/L, potassium=5.3 mEq/L, urea=14 mg% and creatinine=0.4 mg%. An arterial blood gas (ABG) showed pH=7.4, pCO2=31 mmHg, pO2=93 mmHg and HCO3−=20.6 mmol/L. Two peripheral (left lower and upper limbs) iv. cannulae were secured. Antibiotics, antipyretics and intravenous fluids were started. She developed hypotension the evening prior to surgery. Infusion of dobutamine 18µg/kg/min and dopamine 20µg/kg/min was started.
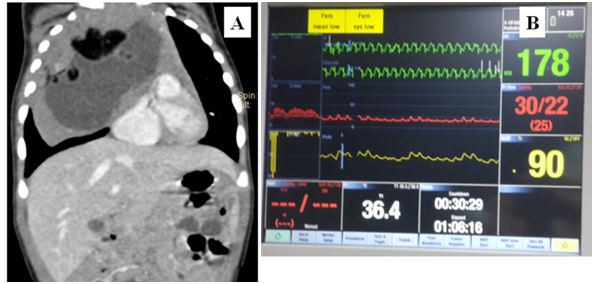
Figure 1 Computed-tomography, intraoperative haemodynamic fluctuations. (A) Anterior mediastinal mass compressing the right lung, bronchus and cardia. (B) Haemodynamic extremes.
On the morning of surgery she was irritable, HR=78 b/min, BP=60/30 mmHg and RR=80 breaths/min. Equipment for managing difficult airway including hollow bougie, rigid bronchoscope and jet ventilation were kept ready. Presence of the cardiothoracic surgeon was ensured. In the operating room, she was placed in left-lateral position. Electrocardiogram, noninvasive-BP and pulse-oximetry monitoring was initiated and pre-oxygenation begun. GA was induced with i.v. fentanyl=10 µg, propofol=10 mg and succinyl choline=10 mg. IPPV with face mask was ineffective. A 3.0 mm cuffed endotracheal-tube was secured on second attempt. There was bradycardia (HR=64 b/min) about 3 min after induction. Following intubation, the peak airway pressure was 23 cmH2O and end-tidal CO2 (ETCO2)=80 mmHg. HR improved soon and pressure-controlled IPPV was started.
Thereafter, the baby was placed in supine position. Anaesthesia was maintained with sevoflurane (end-tidal concentration=2.0-2.6%) in oxygen-air mixture. She received a total of fentanyl=60 µg, and atracurium=0.5 mg repeated as needed. A 4Fr double-lumen right internal jugular central venous catheter (CVC) and 22G femoral arterial cannula were secured under ultrasound-guidance. Invasive-BP monitoring was commenced. Inotropes were infused via the lower limb venous access as the extent of encasement of the vessels by the tumour was uncertain and lest a need arose to take control of the superior vena cava. Thoracotomy was performed via a clam-shell incision. There were episodes of hypotension (Figure 1B) and low tidal-volume delivery during tumour mobilisation due to compression of major vessels and lung. The surgeons were informed and pressure was released intermittently. The BP and tidal-volume delivery stabilised after excision of the tumour (size=10x8x7 cm). Inotropes were discontinued by the end of surgery. An ABG performed towards the end of surgery showed pH=7.36, pCO2=35 mmHg, pO2=174 mmHg and HCO3−=19.8 mmol/L.
Bilateral intercostal drains were placed and thorax closed. After the surgery, a 21G epidural catheter was inserted in left-lateral position at T6-T7 (19G Tuohy’s needle, space identified by ‘loss-of-resistance-to-saline’) and fixed at 4cm from the skin. Analgesia was commenced with 5ml of bupivacaine-0.1% with fentanyl 5 µg followed by an infusion of bupivacaine-0.1% with fentanyl 2 µg/ml at 2ml/h for the next 48h. Neuromuscular blockade was reversed and trachea extubated when baby was awake. Post-extubation HR was 12 b/min, BP=100/52 mmHg and RR=30 breaths/min. Baby was shifted to intensive-care unit. Epidural analgesia was supplemented with i.v. paracetamol 75 mg Q6h. A chest-radiograph obtained on postoperative day-2 showed a reexpanded right lung. Gradually, her HR and BP normalised, and RR was <30 breaths/min. Nasogastric tube feeding and subsequently, oral feeds were started. Intercostal drains were removed on day-7 and she was discharged on postoperative day-15. The histopathological diagnosis was of a mature teratoma. The child was doing well at six-month follow-up.
In children, the most common masses in the anterior mediastinum tend to be lymphomas (primarily of the Hodgkin’s type), lymphangiomas (cystic hygroma), neurogenic tumours and germ cell tumours (teratomas). Thymomas and thymic cysts are rare in childhood. Other tumours may be Non-Hodgkin’s Lymphoma and Acute Lymphoblastic Leukaemia. Other causes of anterior mediastinal mass include vascular malformations and bronchogenic or enteric cysts.8−11
The incidence of complications related to airway obstruction with the use of GA in patients with mediastinal masses has been reported to be 7% to 18%.9 GA exacerbates extrinsic airway compression by various mechanisms.
Concerns in this case were anaesthetising an infant with impending cardiorespiratory collapse, induction of GA, site of venous access, invasive-BP monitoring, haemodynamic fluctuations, potential major haemorrhage and postoperative analgesia.
Faced with an impending cardiorespiratory collapse because of the pressure effect of the tumour and septicaemic shock, we had to decide whether to proceed with the surgery or not. Because of the worsening haemodynamics, we proceeded with surgery rather than wait for the patient to stabilise. Withdrawal of inotropes at the end of surgery showed that haemodynamic deterioration the night before surgery was mainly due to mechanical reasons.
We preferred intravenous induction of GA as it is the quickest method to secure airway and there was no stridor indicating fairly open larger airways. We intubated in left-lateral position as this relieves the airway obstruction by taking some weight of the tumour off the tracheobronchial tree. Inability to mask ventilate was possibly because of the collapse of the airway due to mass effect. Intubation was immediately attempted as mask ventilation was ineffective. Fortunately, intubation was successful and we did not have to resort to rigid bronchoscopy which was our alternative plan. We did not administer vagolytics to counter bradycardia as we thought it to be due to hypercarbia and hypoxaemia. The high ETCO2 (80 mmHg) soon after intubation confirmed this and the heart rate improved thereafter once effective ventilation was achieved. Epidural was not placed preoperatively due to the haemodynamic instability, possibility of major haemorrhage and the need for urgent tumour excision.
Even though we had secured a CVC, ionotropes were infused via the venous access in the lower limb as the exact extent of encasement of the tumour over the vessels were yet uncertain and it would have been difficult to change the site of drug administration intraoperatively had a need arose to take control of the superior vena cava. Invasive blood pressure monitoring provided us real time haemodymanics and a better titration of ionotropes. The surgical team was provided timely accurate feedback which helped to manage the patient better.
Guidelines, reviews, large series of cases14 and parameters to predict risk15 have been published for the management of patients with mediastinal mass. However, the local availability of resources and expertise often dictate the management of such patients.
We could successfully manage this infant of mediastinal mass who presented with imminent cardiorespiratory collapse because of careful decision making, availability of equipment and expertise needed to manage such complicated cases and also a team work with good communication between the care givers.
The study was supported by department resources and no external funding received.
None.
Author declares that there is no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.