Journal of
eISSN: 2373-6437


Emerging evidence have demonstrated that WISP1, a member of CCN protein family, plays an important role in the manifestation and development of many respiratory diseases, such as lung cancer, pulmonary fibrosis and asthma as well as ventilator-induced lung injury. The production of WISP1 and the following activation of WISP1-mediated Wnt signaling pathways may facilitate and even amplify the pathological processes of the diseases. Toll-like receptors and integrins are also participated in the signaling pathways. This review focuses on the impact and mechanism of WISP1 in pulmonary diseases and proposes that WISP1 holds promise as a diagnostic marker and/or therapeutic target.
Keywords: WNT1 inducible signaling pathway protein 1, WISP, CCN, lung disease, wnt signaling pathway, ventilator-induced lung injury, toll-like receptors, integrins
WNT1-inducible signaling pathway protein 1 (WISP1), also known as CCN4, is a member of the cysteine-rich CCN family of growth factor proteins. Cysteine-rich angiogenic protein 61 (CYR61/CCN1), connective tissue growth factor (CTGF/CCN2), and nephroblastoma over expressed protein (NOV/CCN3) were the first discovered proteins in the family, so the acronym CCN stems from them; together with three WNT-induced secreted proteins, they comprise the CCN family of matricellular proteins.1,2
The CCN protein family includes:
CCN1= CYR61 (cysteine-rich angiogenic protein 61)3
CCN2= CTGF (connective tissue growth factor)4
CCN3= NOV (nephroblastoma overexpressed)5
CCN4= WISP1 (WNT1-inducible signaling pathway protein-1)2
CCN5= WISP2 (WNT1-inducible signaling pathway protein-2)6
CCN6= WISP3 (WNT1-inducible signaling pathway protein-3)7
The CCN protein family secrets extracellular matrix (ECM)-associated proteins and is related to a variety of important cell function pathways, including mitosis, chemotaxis, adhesion, migration, survival, and differentiation, as well as cartilage formation, angiogenesis, tumor formation, and wound healing. CCNs have also been implicated in many human diseases.8-10 WISP1/CCN4 is a member of the CCN protein family. Abnormalities of the WISP1 signaling pathway lead to a variety of pathological phenomena, such as fibrosis, osteoarthritis, and even cancer. Many respiratory diseases, such as pulmonary fibrosis, lung cancer, pulmonary inflammation, and ventilator-induced lung injury (VILI), are also associated with the WISP1 protein. The role of WISP1 in the occurrence and development of disease are reviewed.11,12 Here, we focus on the impact of WISP1 in pulmonary disease and summarize recent studies in which WISP1 has been shown to hold promise as a diagnostic marker and/or therapeutic target.
A classical CCN protein contains an N-terminal secretory signal peptide and four functional domains:
A full length WISP1 consists of four modules: insulin-like growth factor binding domain (IGFBP) in red, von Willebrand factor C repeat (VWC) in blue, thrombospondin type-1 repeat (TSP-1) in yellow, and cysteine knot (CT) in green. The protein is split into two halves separated by a variable ‘hinge’ region. Different binding partners of each module are also depicted: insulin-like growth factors (IGFs); bone morphogenic protein 4 (BMP4); transforming growth factor β (TGF-β); LDL receptor protein 1 (LRP-1); and heparin sulphated proteoglycans (HSPGs).13
Variation in CCN protein structure is related to the loss of one or more domains; the loss of different domains will result in different biological functions and ultimately lead to diseases.2 A full length WISP1 consists of four modules. Some studies have confirmed that invasive scirrhous gastric carcinoma14 and cholangiocarcinoma15 are related to the deletion of a module named VWC, reduced by alternative splicing of exon 3; WISP1 without the VWC module is referred to as WISP1v. Furthermore, besides full-length WISP1 and WISP1v, loss of more domains can be found in two hepatocellular carcinoma cell lines and a human chondrosarcoma-derived chondrocytic cell line, including ex 3-4 deltaWISP116 and WISP1vx.17 Models of all described WISP1 variants are shown in (Figure 2).18
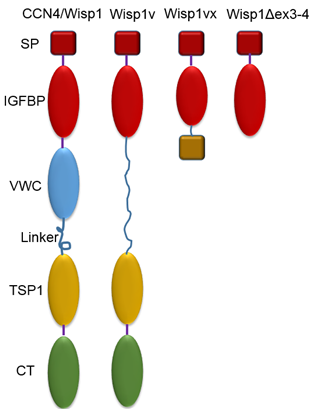
Figure 2 Normal and abnormal molecular structures of CCN proteins: full length CCN4/WISP1 and truncated variants.
The full length WISP1 protein consists of 367 amino acids with a predicted molecular mass of 40 kDa and has 38 conserved cysteine residues and four potential N-linked glycosylation sites.11,19 In fact, observations have shown that WISP1 is glycosylated, and the glycosylation patterns of WISP1 differ between types of cancer cells and healthy fibroblasts.20 In addition, due to the lack of mammalian post-translational modifications, over expressed WISP1 in mammalian cells and recombinant WISP1 produced in Escherichia coli produce different biological effects on cells.21 Based on these results, post-translational modifications seem to affect WISP1 function.
WISP1v is WISP1 with deletion of a VWC module reduced by alternative splicing of exon 3.14 WISP1vx lacks VWC and TSP1 domains and part of the IGFBP domain (23 bp shorter than the full-length exon). The IGFBP/CT fusion coding frame is not translated properly after the alternative splice site because of a frame-shift. The protein product is a single IGFBP module, in which eight C-terminal amino acid residues are removed, and an extra 14 residues are added in their place.17 WISP1Δex3-4 splice variant is a product of joining exons 2 and 5 with a frame shift that leads to a premature stop. As a result, the predicted protein has only the first module.16 SP: signal peptide, IGFBP: insulin growth factor binding protein, VWC: von Willebrand Factor C, TSP1: thrombospondin type 1 repeat, CT: C-terminal domain.
Expression of WISP1 in disease
WISP1 exists in many tissues and organs, such as epithelial tissue and the heart, kidney, lung, pancreas, placenta, ovary, small intestine, spleen, and brain,22 so it is related to the occurrence and development of many diseases. Cerneaet al.23 stated that WISP1 can be used as a new target gene for bone morphogenetic protein -3 (BMP3), and it was found that the BMP3/WISP1signaling pathway plays an important role in the proliferation of mesenchymal stem cells and the process of lipid formation.23
WISP1 has also been demonstrated as a possible target gene to treatesophageal squamous cell carcinoma. Zhang and his team found that WISP1 can enhance its own expression in response to radiation and form a positive feedback loop through which cancer cells increase their ability to resist radiation. So it can be considered a potential target for improving the sensitivity of esophageal cancer patients to radiotherapy.24
Concurrently, the expression of WISP1 has been found to be higher in breast cancer cells that in normal breast tissue, and over expression of WISP1 inhibited the breast cancer tumor suppressor gene NDRG1.25 WISP1 also plays a role in the growth and metabolism of bone. WISP1is a negative regulator of osteoclast differentiation, which plays multiple roles in controlling bone homeostasis.26 Subsequently, WISP1expression was found in osteoblasts and in the perichondrial mesenchyme by using a combination of in situ hybridization and immunohistochemistry.27 We may consider WISP1/CCN a prognostic marker in certain diseases such as pancreatic ductal adenocarcinoma and lymph nodemetastasis in oral squamous cell carcinoma.28,29
WISP1has also been found to play a role in many pulmonary diseases. Gavin BJ et al.30 first reported WISP1in the lungs in 1990.30 WISP1was then also found in various tissues and organs and was found to be expressed in various types of cells; thus, studies on WISP1have attracted increasing attention.19 Diseases with increased morbidity and mortality such as pulmonary fibrosis and lung cancer are still hot topics in respiratory disease research.
The most challenging therapeutic regimen issues could be solved if a biomarker could be found to represent a potential downstream mediator for therapeutic intervention in pulmonary fibrosis. Recently, studies on WISP1in pulmonary fibrosis has increased. Stephan Klee et al.31 reported that WISP1expression was regulated by several profibrotic growth factors and that canonical signaling and ALK4/5/7 play critical roles in WISP1expression induced by TGFβ.31 Also, in the course of pulmonary fibrosis, the expression of WISP1induced by TGF-β1 is regulated by miR-92a.32 Different types of lung cells will produce different effects under recombinant WISP1pretreatment. Pretreatment of type II airway epithelial cells (AECs) led to increased proliferation of type II AECs and epithelial-mesenchymal transition, whereas treating fibroblasts enhanced the deposition of the ECM.33,34 Interestingly, neutralizing monoclonal antibodies specific for WISP1attenuated bleomycin-induced lung fibrosis in mice.33
Several WNT signaling proteins, including WNT1, WNT2, and WNT7A, are differentially expressed in lung cancer cells. WNT1 is related to lung cancer.35,36 He et al.36 reported that cancer cells expressing WNT1 are resistant to apoptotic therapies.36 In contrast, anti-WNT1 monoclonal antibodies can suppress tumor growth in vivo.35 As a WNT1 wingless pathway target gene, alterations of WISP1have been reported in lung cancer specimens.20,37,38 Usually, tumor progression has been associated with WISP1expression; expression of WISP1in lung cancer cells was significantly higher compared with healthy lung tissues. Chen et al.37 and Yang et al.39 found that the expression of WISP1in lung adenocarcinoma was significantly higher than that in healthy lung tissues, but they did not find a correlation between WISP1level and prognosis.37,39 The gene polymorphism of WISP1may also be used in the study of patients with lung cancer. Chen et al.40 recruited 556 patients with lung cancer and 254 healthy controls and their results showed that several genotypes of WISP1were associated with susceptibility to lung cancer and several WISP1genotypes were significantly related to the efficacy of platinum-based chemotherapy in lung cancer patients. This finding can be used to predict the toxicity of platinum-based chemotherapy in lung cancer patients.41 The emergence of various studies39,40-42 on WISP1may reveal it to be a novel and useful biomarker for the diagnosis and treatment of lung cancer.
Asthma is a chronic inflammatory disease. Previous research has focused on pro-survival and pro-fibrogenic signaling pathways, which are closely related to the remodeling of airway tissue. Along with further research, the WNT signaling pathway has been considered promising to further explore the molecular mechanism of organ fibrosis and tissue remodeling. Trischler et al.43 reported that activation of the WNT signaling pathway, especially WISP1, is related to the airway remodeling process.43 Both Sharma and Yang reported that WISP1expression was correlated with asthma airway remodeling.44-46
WISP1is also involved in acute lung injury (ALI). The extensive use of anesthesia ventilators has contributed to an increase in VILI. The gene-encoding proteins of the CCN family, especially WISP1, are extremely sensitive to changes in the environment including mechanical stretch,2 however, the specific mechanism of the protein in various stretch-induced lung injury is not clear. Li and colleagues demonstrated that WISP1/CCN4, identified by a genome-wide approach, acts as a cellular accessory molecule that leads to VILI in mice.47 Alveolar-capillary permeability, which can be used to determine the extent of lung injury, is actually proportional to WISP1secreted in vivo after high tidal volume ventilation. Heise48 found that WISP1is significantly up-regulated in stretched type II epithelia in a hyaluronan-and MyD88-dependent fashion; meanwhile, the epithelial mesenchymal transition in stretched cells can be prevented by using WISP1antibody. Faisyet al.49 have also identified that stretch led to significantly higher mRNA levels of WISP1.
In addition to the correlation between WISP1and pulmonary disease, the expression of WISP1has been observed during lung development. Sharma et al.45 confirmed that the WISP1gene was associated with intrauterine airway development.
WISP1and the WNT pathway
WISP1has been suggested to act as a putative downstream effector of the WNT pathway.19 The WNT signaling pathway is activated via two distinct branches: the canonical and non-canonical pathways, based on the expression profiles of receptors, co-receptors, and the activity of intracellular WNT signaling regulators.50,51 The hallmark of the canonical WNT/β-catenin pathway is that it activates the transcription factor β-catenin, a downstream effector of the pathway that is initiated by WNT ligands to form a Frizzled receptor and low density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptor complex that inactivates glycogen synthase kinase-3β (GSK3β) to block β-catenin phosphorylation and degradation that leads to accumulation of hypophosphorylated β-catenin in the cytoplasm and subsequent translocation to the nucleus, where it regulates target gene expression through interactions with a family of transcription factors.52-54
Actually, the functional β-catenin/TCF heterodimeric transcription factor has been visualized in vivo, where β-galactosidase has been placed downstream from promoter elements harboring canonical TCF cis elements (e.g., TCF-optimized promoter-LacZ or TOPGAL mice).55 These TOPGAL mice have provided a sensitive approach for dissecting the role of the canonical β-catenin pathway in lung development,56 injury,57 and repair,58,54 as well as airway epithelial lineage and stem cell studies.59 Pharmacological approaches to dissect the contribution of WNTβ-catenin canonical signaling include activation by lithium chloride, a well-known inhibitor of GSK-3β,58 or inhibition by using ICG-001, a selective inhibitor of WNTβ-catenin-dependent transcription.60 Recently, the convergence of WNT/β-catenin canonical signaling, WISP1, and lung epithelial cell repair was demonstrated after inflammatory lung injury.61 Extrapolation of the reparative role of WISP1needs to put into context, as reviewed by Lawson and Blackwell.62 Li47 noted that WISP1enhances alveolar capillary permeability in ALI and Konigshoff et al.63 demonstrated that anti-WISP1antibodies attenuated bleomycin-induced lung fibrosis. Fewer reagents and progress in the lung with respect to the non-canonical pathway is apparent, although detection of hallmark regulatory proteins WNT5A or WNT11 suggests this pathway may be operative in certain forms of lung cancer.64 Although the original observations by Slutsky et al.65,66 concluded that WNTβ-catenin signaling is important in VILI, they reported increases in indices of activation of both non-canonical (WNT5A) and canonical pathways in whole rat lung. Further confirmative studies are required to identify which WNT signaling pathway is responsible for WISP1production in the lung.
WISP1and toll-like receptor (TLRs), integrin-mediated signaling pathway
Mutual connections between WISP1and TLRs and integrin are fairly complicated because of the wide variety of TLRs and integrins. The occurrence and development of many diseases are related to these connections. WISP1(CCN4) is one of the CCN family proteins; the CCN proteins are key signaling and regulatory molecules involved in many vital biological functions, including cell proliferation, angiogenesis, tumorigenesis, and wound healing.67
CCN proteins interact with cell surface integrins (e.g. cysteine-rich protein 61 (CCN1) via αvβ3,68 CCN3 via αvβ5,69 and WISP1(CCN4) via αvβ370 to induce intracellular signaling events.2,8 Integrins appear to regulate inflammatory responses such as TNF release.71 Indeed, RGD- (Arg-Gly-Asp-Ser peptides) sensitive integrin signaling in VILI72 and αvβ3 and αvβ5 in particular have been identified to play critical roles in regulating pulmonary permeability in ALI and VILI.73
Sheppard et al.74,75 have demonstrated that β3 is protective (i.e.,β5-null mice are sensitive) to endotracheal and intraperitoneal LPS and cecal ligation and puncture (CLP), whereas Pittet et al. have shown that β5 enhances (i.e.,β5-null mice are resistant) to lung vascular leak after infection,76 ischemia/reperfusion, or VILI.77 Meanwhile, a new publication by Ding78 suggested that the integrin family member β-6 is known to play an important role in regulating lung inflammation, macrophage protease expression, and pulmonary edema during the process of ALI. In this process, both WISP1and integrin β6 constitute a pathway to regulate pathophysiological process in the lung. Also, RGDs, which act as an inhibitor of integrin-ligand interactions, can block the pathway to alleviate ALI induced by CLP and improve the survival rate of mice.
Activation of the TLR complex, a receptor of the innate immune system, may underpin the pathophysiology of many human diseases, including asthma, cardiovascular disorders, diabetes, obesity, metabolic syndrome, autoimmune disorders, neuro-inflammatory disorders, schizophrenia, bipolar disorder, autism, clinical depression, chronic fatigue syndrome, alcohol abuse, and toluene inhalation.79 TLRs play a pivotal role in the innate immune response in sensing and responding to cellular injury in the lung.80
TLR4 is the most important transmembrane protein receptor in the TLR1-9 family that activates the cellular inflammatory reaction by interacting with CD14 extracellular membrane,81 and transmitting biochemical signals through the MyD88 pathway82 and TRIF intracellular pathway.83 TLR4 has been shown to play a critical role in ALI induced by high tidal volume mechanical ventilation (HTV),82,84,85 LPS,86 acid aspiration,87 hemorrhage,88 and ischemia and reperfusion injury.89 Hu et al.84 showed that HTV increases WISP1expression;84 meanwhile, mechanical stretch has been demonstrated to increase endogenous TLR4 ligand production and activate TLR4 in healthy mice.90,91 Several studies have shown that TLR4 is associated with VILI in animal models.82,84,90 Zhang’s et al.47 found that HTV can increase the expression and production of WISP1, which might contribute to VILI in mice; such a process probably occurs through modifying and/or enhancing TLR4-mediated cellular functions because the interaction between WISP1with TLR4 is synergized. This includes both increased WISP1production in HTV and activation of TLR4 signaling, leading to further lung injury.
As a potential proliferative and restorative protein, WISP1has demonstrated great promise for the development of novel therapeutic strategies against acute and chronic disorders that involve the nervous, musculoskeletal, cardiac, pulmonary, and vascular systems.92 Meanwhile, with the development of research onWISP1in pulmonary diseases, more and more biological functions of WISP1have been found, which can produce complex biological outcomes. Under certain conditions, WISP1plays a primary role during the occurrence and development of pulmonary disease. Emerging studies demonstrate that targeting CCN protein expression or signaling pathways holds promise for the development of diagnostics and therapeutics for pulmonary diseases. Nevertheless, many questions remain to be answered, such as: In the lung, where and which cell type is the major source of WISP1production? Which pathway, the non-canonical or the canonical WNT pathway, is the main productive route? How can it be regulated? Accordingly, identifying the role of WISP1in pulmonary disorders is essential to effectively target this pathway for clinical therapies and diagnostic prevention.
This research was supported by NIGMS R01GM108639-01A1 grant 1151456 to Zhang. We thank Ms. Christine Heiner, Scientific Writer in the Department of Anesthesiology and Surgery at the University of Pittsburgh, for assistance with scientific editing that greatly improved the manuscript. We would also like to show our gratitude to Ms. Karla Woosloose, laboratory manager for providing comments on an earlier version of the manuscript.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.