Journal of
eISSN: 2572-8466


Mini Review Volume 5 Issue 3
1Universiti Teknologi Malaysia, Malaysia
2Department of Environmental Engineering, Universiti Teknologi Malaysia, Malaysia
3Department of Building and Construction Engineering, Universiti Tun Hussein Onn Malaysia, Malaysia
4Faculty of Engineering Technology, Universiti Malaysia Pahang (UMP), Malaysia
5Biological and Ecological Engineering, Oregon State University, USA
Correspondence: Mohd Fadhil Md Din, Department of Environmental Engineering, Faculty of Civil Engineering, Universiti Teknologi Malaysia, Johor Bahru 81310, Malaysia, Tel 6019?7347878, Fax 607?5533149
Received: March 05, 2018 | Published: May 25, 2018
Citation: Krishnan S, Din MFMD, Taib SM et al. Utilization of micro -algal biomass residues (MABRS) for bio -hythane production -a perspective. J Appl Biotechnol Bioeng. 2018;5(3):166-169. DOI: 10.15406/jabb.2018.05.00133
This contribution presents the technical possibilities for hydrogen and methane production from micro‒algal biomass residual wastes. Algal biomass is rich in carbohydrates which can be utilized as a promising source of substrate for dark fermentation. It becomes more significant when biomass is produced by capturing atmospheric greenhouse gas, CO2. In the present technical note, how clean energy can be generated in the form of bio‒hydrogen and methane utilizing algal biomass residues is discussed shortly. The scientific contribution of this two‒stage technology may play a significant role in degrading micro algal biomass in to zero waste and developing an energy‒efficient strategy for waste management.
Keywords: clean energy, micro‒algal biomass residues, hydrogen, methane, waste management
The world is facing the challenges of high energy demand with the escalating fuel prices due to global population and industrialization. Energy from biomass (plants, energy crops, biomass wastes, etc.) would contribute to a stable energy supply and to local society due to an increase in commercial activities. Microalgae offer a great promise as a renewable oil sources for biodiesel production. It’s estimated that for each billion gallons of algal biofuels generated, 4 to 5 million tons of micro‒algal biomass residues (MABRs) are generated.1 Since the economics of micro‒algal biofuels are challenging, it is important to recover the energy, carbon, and nutrients retained from its residue. This “residual waste” could be a major cost burden, environmental problem, or the key to enhancing profits from biofuel production. There are several ways in which MABRs can be used to their full potential to produce value added products (VAPs). Such approaches for the application of residual carbohydrates, proteins, etc., cannot find cost effective separation technologies, and as well as viable markets.2 But these residues can be seen as an attractive feedstock for the production of energy fuels. In particular, the MABRs are considered to be a prominent substrate for bio‒hydrogen fermentation. Therefore, the conversion of MABRs into biogas by dark fermentation could serve the dual role of renewable energy production and sustainable development of the microalgal biodiesel industry.
The dark fermentation is a fermentative conversion of organic substrate into hydrogen by acidogenic bacteria through the acidogenesis pathway, without light energy requirement.3 While, anaerobic digestion is biological processes that can be used to treat waste/wastewater and recover methane via methanogenesis route from waste/wastewater using methanogenic bacteria and archaea consortium.4 In the traditional one stage process hydrogen is usually not detected as hydrogen is consumed during methanogenesis to produce CH4 and CO2 as end product. Two‒stage process has been used for bioenergy production, which essentially consists of acidogenic and methanogenic processes.5 In the first acidogenic process, organic polymers, such as carbohydrates, proteins, and lipids, are converted to volatile fatty acids (VFAs) and hydrogen. Subsequently, the effluent rich in VFAs are then converted to methane in the subsequent methanogenic step. The objective of using two‒stage process was to separate acidogenesis and methanogenesis and optimize each process separately. Compared with the traditional one‒stage process, the two‒stage process may offer several advantages, such as enhanced biogas yield, improved digestion efficiency of the substrate, and improved the stability of the process.6 Until now, the importance of MABRs in bio‒hydrogen production has not been studied. In this short technical report, the importance of MABRs in bio‒hydrogen production is discussed. The biochemical pathway, factors affecting the bioenergy production and suitable post treatment is also discussed.
Bio hydrogen from MABRs
MABRs contains large amount of fixed carbon and energy in the form of proteins, carbohydrates, inorganic nutrients (nitrogen and phosphorous) with little or no lignin, which enables easy hydrolysis.7 Bio‒hydrogen surmounts to be an ideal alternative to fossil fuels because of its high energy density by mass, zero combustion products without carbon prints.8 For bio‒hydrogen production, pretreatment seems to be a necessary step for MABRs, because the complex oil extraction processes caused them to resist biodegradation which included dewatering, drying, destruction of the cell wall and extraction with solvent.9 Pretreatment methods for substrates and seed sludge, such as mechanical, chemical, thermal pretreatment or their combinations, have proved to be useful for enhancing hydrogen yields. Chen & Oswald10 found that the thermal pretreatment of green algal biomass induced a 33% improvement in methane production.10 Most importantly the adequate loading and inoculum to substrate ratios must be necessarily maintained during hydrogen fermentation. Dark fermentation is mainly composed of hydrolysis and acidogenesis of anaerobic digestion. Hydrogenase is the key enzyme that catalyses molecular hydrogen formation by combining protons and electrons.11
The carbohydrates present in MABRs are the primary substrates responsible for hydrogen fermentation. Glucose, which is the most abundant and typical hexose, can be stored in the forms of starch, glycogen, cellulose, and trehalose in MABRs.12 The biochemical pathway for glucose conversion in dark fermentation is shown in Figure 1. Glucose is degraded by hydrogen producing bacteria (HPB) into pyruvate, coupled with generation of reduced nicotinamide adenine dinucleotide (NADH). Pyruvate is degraded into lactate (end product) by consuming NADH, or converted into acetyl‒CoA, coupled with generation of NADH and carbon dioxide, or generation of hydrogen and carbon dioxide, or generation of formate. The formate is further degraded into hydrogen and carbon dioxide. Acetyl‒CoA is further converted into end products, such as ethanol, butyrate, and acetate, with or without consuming NADH. NADH+ and H+ generate hydrogen by catalysis of HPB hydrogenase (1mol NADH generates 1mol hydrogen).13 One mole of glucose can theoretically generate 4mol NADH through the acetate pathway. Therefore, 1mol glucose can theoretically generate 4mol hydrogen. However, the production of other soluble metabolite products such as ethanol, lactate, and butyrate consumes NADH, resulting in the decrease in hydrogen yield. The stoichiometric yield of hydrogen from biomass residue is estimated to be 33‒397.8mLH2/g under standard temperature (0°C) and pressure (1atm).14 This reaction takes place under strict anaerobic conditions by HPB.
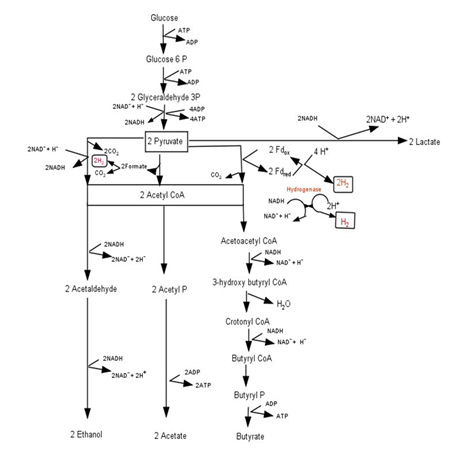
Figure 1 Biochemistry of dark fermentation.3
Factors affecting hydrogen production
The inoculum type (microbial community), reactor configuration, temperature, pH, metal ions such as Fe2+, nitrogen and phosphate are considered the key environmental factors affecting during dark fermentation. The most common hydrogen‐producing strict and facultative anaerobes are Clostridium butyricum, and Klebsiella pneumoniae, Escherichia coli, Enterobacter aerogenes, Rhodospirillum rubrum, Methanobacterium formiccium, respectively. According to Mamimin et al.15 it was possible to use natural anaerobic microflora from sludge, instead of pure culture of isolated strain, to produce significant amounts of hydrogen under anaerobic fermentation in a batch culture.15 Dark fermentation may be divided into mesophilic (e.g., 25–40°C), thermophilic (e.g., 40–65°C), as well as hyper‒thermophilic (e.g., 45°C) conditions.12 Optimal pH Values have been commonly reported in the range of 5.5–6.5 for dark fermentation. The C/N ratio of substrate is an important factor for the yield in dark fermentation. Sompong et al.16 highlighted that a raw palm oil mill effluent (POME) supplemented with nutrients (N, P and Fe) gave a 20% increase in hydrogen production yields as well as 58–61% higher hydrogen contents as compared to raw POME.16 The hydrogen production rate could be increased by 60% if raw POME is adjusted to an optimized iron concentration of 257mg/l, a C/N ratio of 74 and a C/P ratio of 559.8 Until now, very few studies have been reported for bio‒hydrogen production using MABRs through dark fermentation. But, fermentative hydrogen production from the whole micro‒algal biomass with low lipid content has been widely reported. Yan et al.17 reported the hydrogen yield of 105.0–133.0mL H2/g under a mesophilic condition (35°C) using Microcystis sp. (Taihu blue algae) as a feedstock.17 Similarly, Yang et al.9 reported hydrogen yields of 45.5–46.0mLH2/g VS from Scenedesmus sp. biomass under a mesophilic condition (37°C) after alkali pretreatment.9 Further optimization of key experimental factors, genetic modification and metabolic engineering of microalgae are the eventual approaches that make hydrogen and methane production cost‒effective and sustainable.
Suitable post treatment after hydrogen production
However, less than one third of energy in glucose can be converted to hydrogen in dark fermentation, whereas more than two thirds of energy remains in the dark fermentation effluent in the forms of soluble metabolite products. After dark fermentative H2 production, the spent media thus produced containing short‒chain fatty acids such as acetate, butyrate, propionate, etc. could be a suitable substrate for methanogens (Figure 2). Hence, integration of bio‒hydrogen with bio‒methane process under the eponym of bio‒hythane could help in improvement of gaseous energy recovery. The principle enzyme system involved in bio‒methane production is methyl‒coenzyme‒M‒reductases. The energy metabolism of methanogenic archaea proceeds by a stepwise reduction of coenzyme bound and activated C1 intermediates. The central metabolite methyl‒coenzyme M (CH3–SCoM) reacts with the electron donor coenzyme B (HS–CoB) to form methane and the heterodisulfide CoM–S–S–CoB. This reaction is catalysed by methyl‒coenzyme M reductase (MCR). This reaction takes place under strict anaerobic conditions by methanogenic microorganisms (Figure 3). Prior to subjection of spent media for bio‒methanation, the pH of it should be adjusted to a range of 7 to 7.8. Moreover, the dissolved H2 in the media also influences the growth of hydrogenotrophic‒methanogens.18 The combination of hydrogen and methane for vehicular fuel purposes improve the lean flammability range (8‒fold more flame speed) and increase the combustion with an engine.19
The major constraints for the production of MABRs based biofuels are availability of nutrients (especially nitrogen and phosphorous) and CO2, technology and cost of production and harvesting. Moreover, poor digestion, biomass composition, low carbon to nitrogen ratio, ammonia toxicity, consistent design, rational control of operational parameters such as temperature, hydraulic retention time, organic loading rate, and microbial consortium are the key challenges during bio‒hythane production.20 It is worth mentioned that the packed bed reactors exhibit higher energy consumptions and investment costs, which limit the use of this technology for the cultivation of microalgae for bio‒fuel applications.21

Figure 2 Schematic representation of bio-hythane production.6
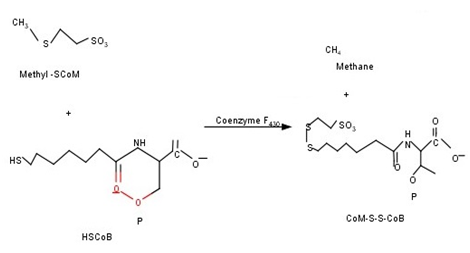
Figure 3 Biochemical reaction involved in methane production.7
Thus, the integration of hydrogen with methane production will benefit the total energy recovery from MABRs. The factors affecting the hydrogen production must be properly optimised for the maximum hydrogen yield. The Development of anaerobic bacterial strains producing mainly acetic and butyric acids with no lactic, propionic acids and alcohol production through metabolic and genetic engineering techniques will improve the biogas yield as well. Additionally, the elimination of H2 consuming homoacetogens from the inoculum and fermentation media by proper pretreatment methods enhances hydrogen yield during acidogenesis step. Co‒digestion of MABRs with other energy rich waste materials, such as forestry residues and agricultural wastes, should be further considered to obtain a balance of C/N ratio, allowing for improving methane yield and counteracting ammonia inhibition.
None.
The authors declare that there is no conflict of interest.

©2018 Krishnan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.