Journal of
eISSN: 2572-8466


Review Article Volume 10 Issue 1
California State University Channel Islands, USA
Correspondence: Bill Tawil, California State University Channel Islands, USA
Received: February 02, 2023 | Published: February 13, 2023
Citation: Vlashi F, Tawil B. Tumor-infiltrating lymphocyte therapy: an overview. J Appl Biotechnol Bioeng. 2023;10(1):32-35. DOI: 10.15406/jabb.2023.10.00324
Cancer immunotherapy has increasingly become an important player in cancer therapy over the years.1 These therapies include T-cell-based therapies, which involve adoptive transfer of autologous T cells derived from the tumors or peripheral blood of cancer patients, vaccines, oncolytic virus therapy, and immunomodulatory antibodies.2 Adoptive T-cell therapy (ACT) is a type of immunotherapy that uses autologous ex-vivo expanded T cells, more specifically tumor-infiltrating lymphocytes (TIL), adoptively transferred back into patients.3 Compared to other ACTs, like CAR-T and TCR-T, TIL therapy has the advantage of having multiple T-cell receptor (TCR) clones which can act against self-antigens and tumor-specific neoantigens.4
ACT using autologous tumor-infiltrating lymphocytes is one of the most effective ways to treat patients with metastatic melanoma and has been shown to facilitate tumor regression in at least half of patients.5 TIL-based therapies have primarily been evaluated in melanoma, mostly because melanoma biopsies are easy to obtain, and melanomas are immunogenic tumors.6 Although, TIL therapy has traditionally been used for the treatment of metastatic melanoma, it has currently shown promising results in other solid tumor indications, such as cervical cancer, ovarian cancer, renal cancer, lung cancer, breast cancer, gastric cancer and head and neck cancer. This expansion of ACT to other tumor indications is largely due to new approaches like genetically engineered T-cells and other methods of antigen targeting.7 Lymphodepletion prior to ACT is a pivotal part of this treatment because it allows for the elimination of T regulatory cells and lymphocytes which can compete with the infused cells for homeostatic cytokines.
In this review, we will provide an overview of the basic premise of TIL therapy and underlying biology of the target cells. We will focus on the history of TIL therapy and the current advancements in the field, including unmodified and genetically modified TIL therapy as well as address the market size for this type of therapy.
T-Cell biology and tumor microenvironment
Tumor-infiltrating lymphocytes are the major cells targeted and expanded in adoptive cell therapy, better known as TIL therapy. These T-cells have become the focus of immunotherapy based on their potent tumor-killing capabilities TILs play an important role in anti-tumor immunity but different types of T-cells, such as cytotoxic T cells, helper T.8 cells, and regulatory T cells also play a role in T-cell mediated responses in the tumor microenvironment (TME). A successful anti-tumor immune response requires the presence, activation, and co-stimulation of these lymphoid components, including CD8+ T cells, CD4+ T cells, B cells and innate lymphoid cells.9 Tumor antigen-specific cytotoxic CD8+ TILs expressing T-cell receptors account for the anti-tumor effects of the immune system, including the specific cell types of CD8+ effector T cells [40]. CD4+ T cells were believed to support the development and function of CD8+ T cells, but recently it has been proposed that CD4+ T cells also exert anti-tumor activity through direct killing and cytokine release.10
While the presence of TILs is associated with better clinical outcomes, the type and function of TILs as well as the TME localization of different TILs are key factors in determining eventual tumor control or progression. The ability of TILs to kill tumor cells will be inhibited by factors in the TME, like regulatory T-cells (Tregs), myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages in addition to immunosuppressive molecules and metabolites.11,12
Current advancements in TIL therapy
The first studies using the adoptive transfer of TILs expanded in Interleukin-2 (IL2) were performed in mice that bore micrometastases from various tumor types.13 These studies showed that TILs were 50 to 100 times more effective in their potency than lymphokine-activated killer cells. In this study, the combination of cyclophosphamide, TIL and IL2 in the mice bearing the adenocarcinoma showed a 100% cure of advanced hepatic metastases and up to 50% cure for advanced pulmonary metastases. The first in-human series of Phase I/II clinical trials using a TIL therapy treatment were performed by Rosenberg and colleagues in the late 1980’s and early 1990’s at the NIH, where autologous TILs were infused into patients in conjunction with lymphodepleting conditioning and high-dose IL2 administration.14,15
Although there is no TIL therapy currently approved by the FDA, there are currently ongoing studies in clinical trials to test the efficacy of this therapy in treating solid tumor indications.16 In a phase II open-label, single-arm, multicenter study in patients with advanced melanoma who have previously been treated with checkpoint inhibitors and BRAF + MEK targeted agents, Lifileucel showed an overall response rate of 36% with two complete responses and 22 partial responses.17 One retrospective analysis report demonstrated that between 2011 and 2019, 21 patients with histologically confirmed metastatic cutaneous melanoma and no standard-of-care treatment options underwent TIL therapy treatment. Results for this study showed a 67% overall response rate, 19% complete response, 86% disease control rate and a 21.3-month median overall survival. In total, 5 patients had durable ongoing responses greater than 30 months post-infusion and the patients who had a complete response remained alive and disease free. One of the limitations of TIL therapy is the dependence on high doses of IL2 which can cause toxicities to the patients.18 To address this issue, one pilot trial tested the infusion of the TIL product followed by low-dose IL2 and saw that complete and durable responses were achieved even at low doses of IL2.
The positive results of TIL therapy in melanoma have encouraged multiple centers worldwide to conduct studies to either reproduce of optimize this process.19 The current first line of treatment for patients with advanced melanoma is treatment with PD-1 blockade or an anti-PD-1 based combination therapy. The use of TIL in combination with anti-PD-1 is currently a subject of clinical trials in addition to the 22 other clinical trials currently evaluating the optimal treatment forms for TIL production. In recent years, there have been 79 trials of TIL therapy (Figure 1A), including 22 kinds of TIL products between 2011 and 2020, in which 54% of the trials are open, 17% are completed and the remaining are terminated or closed, with over 50% of the trials conducted in the USA and 15% in China (Figure 1B).20
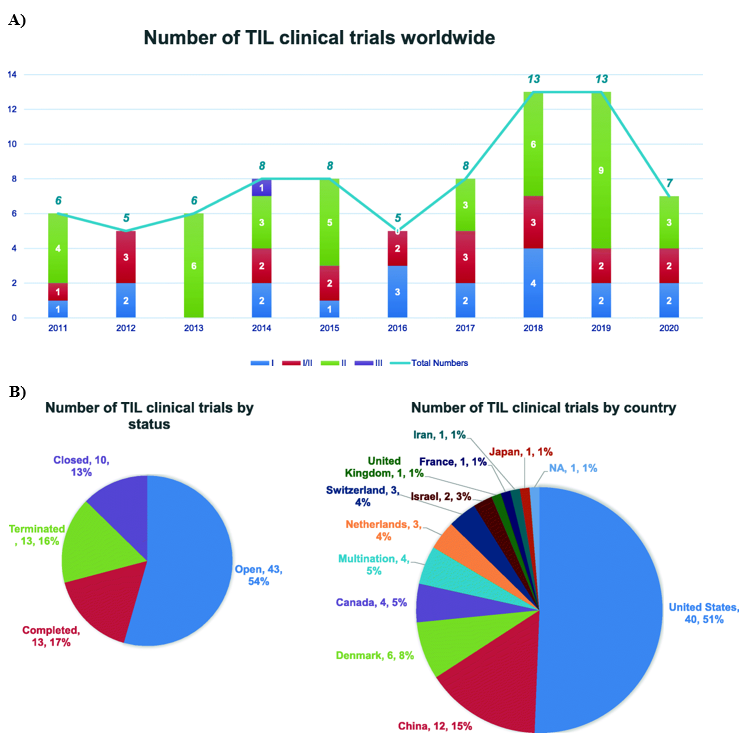
Figure 1 ThreeOverview of TIL Therapy Trials. (A) Distribution of Phase I/II/II TIL Therapy clinical trials from 2011 through 2020. (B) Number of TIL clinical trials by trial status and number of clinical trials by country.34

Figure 2 Three(A) Process of unmodified TIL Therapy (B) Process of engineered T cells.27
Genetically modified TIL therapy
While traditional TIL therapy with autologous unmodified TILs has shown efficacious results, there have been efforts to genetically modify TILs to increase their effectiveness and to include other cancer types. An overview of the differences between traditional unmodified TIL therapy and genetically modified ACT is highlighted in Figure 2.21 TIL therapy involves the harvesting lymphocytes from a patient’s tumor and culturing to encourage T cell growth with IL2 and reinfusion back into the patient. Engineered ACT involves the isolation of T cells followed by infection and transduction with a recombinant virus to express a specific T cell receptor. ACT therapy using T-cell receptor or chimeric antigen receptor (CAR)-retargeted T cells, like the anti-CD19 CAR-modified T cell treatment, resulted in remarkable responses in patients with acute lymphoid leukemia.22
Because of this success with genetically modified T cells with CAR in hematological cancer indications, there has been hope that similar results can be seen in solid tumor indications. Genetic engineering allows T cells to be modified to include new properties and enhanced functions.23 The first step in the development of a TCR gene transfer is the choice of the gene transfer method.24 For ACT trials, this is almost exclusively based on viral vector-based expression systems. For optimal adoptive T cell therapy, the preservation of the replicative life span of memory T cells is crucial for long-term immunity and this can be done by preserving CD28 expression, which is associated with long telomeres, on the infused T cells.25 Restoration of CD28 expression also seems to rejuvenate T cells by reconstituting their ability to produce IL2, which induces autocrine proliferation.26
Limitations of the current unmodified TIL therapy is the toxicity related to high-dose IL2 required for TIL survival and the exhaustion of the final TIL product after rapid expansion.27 Grit Biotechnology developed a genetically engineered TIL product, GT202, that expressed a membrane-bound cytokine that is important for T cell activation and effector function. This product showed enhanced anti-tumor efficacy and decreased IL2 dependency both in vitro and in vivo and is currently in trials in China. In another study, researchers genetically engineered TILs to secrete IL2, which exhibited prolonged survival after IL2 withdrawal in vitro but not in vivo.28 One possible reason for this low response rate is a reduction of telomere length in cells due to prolonged in vitro cultures. This indicates that the manufacturing process of TILs is crucial and that these processes need to be optimized to provide the same effectiveness in vivo as in vitro. One technical difficulty in genetically modifying TIL using a retroviral vector is the ability to get many cells transduced.29 One method using the CXCR2 chemokine receptor as the gene of interest was presented by researchers and is currently being used to produce gene-modified TIL for two clinical trials for the treatment of melanoma.
T-cell therapy market
The global T-cell therapy market size was valued at USD 6.4 billion in 2020 with an expected compound growth rate of 20.2% from 2021 to 2028, and a revenue forecast of USD 20.3 billion by 2028.30 In a market report conducted by Research and Markets, it was similarly estimated that the market value would be USD 4.9 billion in 2022 and USD 23.4 billion in 2023.31 However, the high cost and time-consuming manufacturing process have a high chance of dampening the growth of TIL therapy globally.32 The academic cost for TIL-treatment was calculated at the Netherlands Cancer Institute for be about €62,000 for one patient, which calculates to be about the same in USD.33
TIL therapy has had promising results in Phase I/II clinical trials and could potentially become a standard-of-care for late-stage solid tumors, especially advanced melanoma.34,35 Relapsed, refractory and metastatic cancers represent a high unmet medical need and very few options are available for patients who progress or do not respond to immune checkpoint inhibitors.36
However, TIL therapy does have its limitations. To achieve robust anti-tumor responses, the presence of effector T-cells with anti-tumor activity in the tumor is essential but is not always the case in solid tumors. The tumor microenvironment is immunosuppressive and can cause the exhaustion of infiltrating cytotoxic T cells, thus decreasing the ability of cancer cells to be eliminated. Additionally, IL2 is used as a standard of care to promote TIL growth and activity but can also induce systemic toxicity and promotes suppressive Tregs in the TME.37,38 ACT engineering or combination therapy with other agents that help allow ACT to overcome these challenges will be necessary in the optimization of this therapy for solid tumors.39
Although there are limitations to the success of TIL-based therapies, there are opportunities to optimize this process. The ability to expand TILs ex-vivo provides an opportunity to modify and optimize the infusion product with methods like enrichment of tumor-reactive TILs, genetic engineering, and modulation of T cell metabolism.40 It is strongly believed that TIL therapy will have its strongest impact when combined concurrently with other immunomodulatory therapies, based on the idea that these responses will occur over periods of time and invoke multiple immunological compartments.40
None.
There are no conflicting interests declared by the authors.

©2023 Vlashi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.