Journal of
eISSN: 2572-8466


Review Article Volume 9 Issue 2
1Faculty of Agricultural Sciences, National University of Catamarca, Argentina
2Faculty of Agrarian Sciences, National University of the Littoral, Argentina
3National Council for Scientific and Technical Research, Argentina
4Faculty of Agronomy and Zootechnics, National University of Tucumán, Argentina
Correspondence: Di Barbaro Gabriela, Faculty of Agricultural Sciences, National University of Catamarca, Argentina
Received: February 23, 2022 | Published: March 29, 2022
Citation: Gabriela DB, Eleodoro DV, Celia BW. Topinambur (Helianthus tuberosus) and yacon (Smallanthus sonchifolius): nutraceutical crops? J Appl Biotechnol Bioeng. 2022;9(2):41-47. DOI: 10.15406/jabb.2022.09.00283
This review is about two crops, topinambur (Helianthus tuberosus L.) and yacón [Smallanthus sonchifolius (Poeppig & Endlicher) H. Robinson], which due to their properties should be considered as nutraceutical foods. The common characteristics they present are discussed, such as belonging to the same botanical family (Asteraceae), being ancestral crops produced for their different uses (horticultural, forage and industrial), and for generating tubers that store carbohydrates such as inulin and fructooligosaccharides (FOS). In addition, these compounds are considered to have beneficial effects on nutrition and human health, which would allow them to be defined as nutraceutical foods. Therefore, the objective of this review is to contribute to the dissemination of knowledge about the characteristics of topinambur (Helianthus tuberosus) and yacón (Smallanthus sonchifolius) crops, in order to improve their production, consumption and use.
There are many reasons that force us to seek and study strategies that allow us to increase crop productivity and increase food production. The main reason is the continuous increase in the world population, added to the progressive reduction of the cultivable area due to the advance of urbanization, soil erosion and soil contamination due to the accumulation of toxic products, are aspects that make it essential the application of strategies and biotechnologies. Among these strategies, the use of new crops stands out, fundamentally those called nutraceuticals, that is, in addition to providing food, they provide medical or health benefits, including the prevention or treatment of diseases. That is why this work tries to contribute to the dissemination of knowledge about the characteristics of topinambur (Helianthus tuberosus L.) and yacón [Smallanthus sonchifolius (Poeppig & Endlicher) H. Robinson] crops, both with nutraceutical potential, through in order to improve its production, consumption and utilization.
Crops
Topinambur and yacón crops date back to pre-Columbian times and have remained for a long time restricted to very small areas since they are adapted to Andean ecological conditions, where their use is strongly linked to the traditions of the peoples,1 but in recent years the study of these crops for their nutraceutical properties has gained great interest.
Topinambur (Helianthus tuberosus L.)
The cultivation of topinambur (Helianthus tuberosus L.) is also known as tupinambo, pataca, Jerusalem artichoke, topi and sweet potato sunflower, it is native to the central region of North America2 and belongs to the botanical family of the Asteraceae (Table 1).
|
Kingdom |
Plantae |
|
División |
Magnoliophyta |
|
Class |
Magnoliopsida |
|
Order |
Asterales |
|
Family |
Asteraceae |
|
Subfamily |
Asteroideae |
|
Tribe |
Heliantheae |
|
Subtribe |
Helianthinae |
|
Gender |
Helianthus |
|
Species |
Helianthus tuberosus L. |
Table 1 Taxonomy of Helianthus tuberosus
Fountain: https://www.tropicos.org/name/2700862
Topinambur can be considered a multipurpose crop, since its production is carried out for its following uses: horticultural, forage and industrial, in addition in Turkey it is considered a medicinal plant where diabetic people consume the tubers.3
Human nutrition: topinambur is a crop with great potential for human consumption as a horticultural species and for the preparation of food for diabetics and celiacs, due to its inulin content. The tubers can be consumed fresh, they can be transformed into flour for use in pastry.4 Topinambur tubers are considered a "gourmet" vegetable2 for their healthy nutritional qualities and as delicacies, considering that they do not contain gluten. The way to obtain a healthy food for daily consumption using topinambur tubers as raw material has been studied,5,6 as well as the effect of the culinary preparation on the carbohydrate composition, the texture and sensory quality of the tubers.6,7 Its chemical composition, rich in sugars, mainly inulin, allows it to act as an excellent prebiotic.8,9
The feasibility of preparing topinambur puree was determined, which is considered beneficial for health due to its high content of fructans, whose minimum quantified value was 7.4% in the prepared product, and it is estimated that it would be accepted. by the population and produced on an industrial scale as instant puree.6,10 The effect of feeding H. tuberosus tubers was studied and it was determined that its consumption improves glucose tolerance and hepatic lipid profile in rats fed a high-fat diet, so it was concluded that H. tuberosus tubers topinambur exert anti-fat effects in the liver, based on improvements in glucose tolerance and liver lipid profile.11 In addition, topinambur tubers have been used for wine and beer production for many years in France.2
Animal feeding: Topinambur is considered a valuable fodder since the aerial part is used as fodder in the summer season and the tubers in the winter season. The tubers, leaves and stems of H. tuberosus have a wide variety of uses, including as food for different types of livestock: cattle, pigs, goats. For example, it constitutes an important part of the diet of pigs in Cuba12 and the aerial parts of H. tuberosus are considered valuable forage in the diet of sheep.13 The effect of H. tuberosus in the diet of laying hens was studied and it was determined that the production, quality, and cholesterol content of eggs were not affected, and no adverse effect was recorded on the performance and quality of the eggs of the hens.14 A high production of nectar and pollen was also determined in H. tuberosus, which is why it constitutes an attractive food resource for pollinators, since flowering occurs at a time of low food supply for bees and wasps.15
Industrial use: Numerous investigations indicate the potential of H. tuberosus to produce bioethanol and ethanol.16–23 In addition, it has advantages over other crops, mainly due to its high biomass yield. Research on the processing of the biomass of H. tuberosus, allowed the extraction of commercially interesting phenolic acids, as natural antioxidants for food, with pharmaceutical and cosmetological applications, and as fungicides,24 as well as the production of cellulose from topinambur stems.25
Other uses: Helianthus tuberosus can be considered an important source of raw material for various industries. Currently, work is being done on the extraction of different chemical compounds (such as sugars, 5-hydroxymethylfurfural, levulinic acid, inulin, phenolic compounds such as chlorogenic acid, gallic acid, salicylic acid and caffeic acid, as well as terpenes and flavones) and on the development of efficient and low-cost extraction and purification techniques.25–29
Topinambur leaves were found to exhibit remarkable antimicrobial, antifungal, and anticancer activities. Phytochemical studies have revealed that polyphenols, especially chlorogenic acid, have been considered responsible for these benefits for human health. Chlorogenic acid (3-0-caffeicoylquinic acid, 3-CQA), present in topinambur extracts, has recently received significant attention due to its wide spectrum of pharmacological properties that include anticancer, antioxidant, anti-inflammatory, hypoglycemic and hepatoprotective agents, widely used in the pharmaceutical, food and cosmetic industries. It is also a promising compound used as a precursor for the development of drugs that can control the HIV virus, AIDS. The sources of chlorogenic acid are limited; hence the importance of the production of H. tuberosus leaves, as raw material for its extraction29
The antifungal activity of phenolic substances extracted from H. tuberosus leaves was analyzed and their potential use to improve the preservation of stored fruits and vegetables through the development of treatments with new natural antifungals was studied, and it was concluded that topinambur leaves could be a potential source of natural fungicides.30
In Thailand, the content of nutrients and toxic substances in commonly consumed tubers of H. tuberosus was evaluated. This study determined the nutrients, chemical contaminants (insecticide residues and heavy metals) and natural toxic substances (nitrate, nitrite, cyanide, oxalate, phytate and trypsin inhibitor) present in topinambur tubers. All samples contained considerable amounts of fructans and dietary fiber, as well as potassium and iron. All samples had very low amounts of insecticide residues and naturally occurring toxicants (cyanide and trypsin inhibitor, as well as Pb, Cd, nitrate and nitrite, as well as oxalate and phytate31 the composition of foods and establish the safety of their consumption.
Research developed by Willscher et al.,32 indicate that H. tuberosus is a suitable plant for phytoremediation technologies due to its ability to phytoextract heavy metals, such as Mn, Zn, Cd and Ni, in addition to growing in soils at different pH levels (4 to 6). Therefore, they consider it as a promising species to achieve the success of phytoremediation of soils affected by mining and contaminated with heavy metals. Marzec et al.,33 determined that H. tuberosus plants can be successfully used in wastewater treatment plants, achieving high efficiency in the removal of suspended solids and, due to their high potential for biomass production, they can also be exploited as a bioenergy resource. Given that topinambur is a potential raw material for the sustainable production of bioenergy, which includes biofuels such as bioethanol, biobutanol, biogas and others, as well as precursors for the development of medicines, cosmetics, and food, fundamentally, and in sanitation procedures for soils contaminated with heavy metals. For these reasons, topinambur is considered a multipurpose crop and an important raw material for its environmental benefits and agronomic performance.
Botanical description: Helianthus tuberosus is a perennial herbaceous plant with robust, pubescent, and branched stems that can reach 2 to 3 meters in height, with tubers that bear rhizomes. The leaves vary from 3 to 8 centimeters wide and 10 to 20 centimeters long. Mature leaves are rough in texture with coarse hairs on the upper surface and fine pubescence below. They have inflorescences in terminal chapters of yellow flowers and approximately 3 to 8 centimeters in diameter.28,34 (Figure 1).
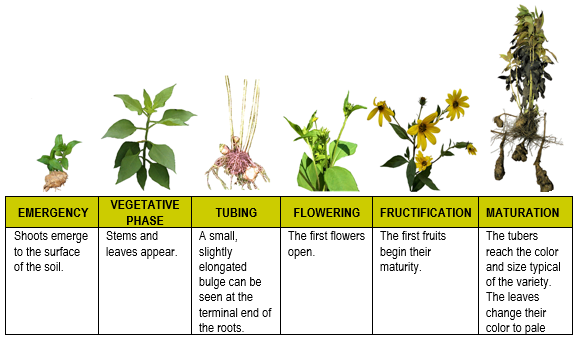
Figure 1 Phenology of topinambur (H. tuberosus) cultivation.72
The roots are fibrous, with short rhizomes that end in a hypogeal cauline tuber. These underground organs are oblong, scaly, and reserve inulin instead of starch.34,35 The tubers vary from knobby to round clusters and have a color range from red to white.36 Cultivated varieties produce white tubers that cluster near the main stem in contrast to wild types that produce elongated reddish tubers at the end of long rhizomes.
Topinambur is a plant with an annual cycle, and the development of the tubers can be summarized as follows: the stolons begin to grow, and the tubers appear ten days later.37 The number of tubers increases until flowering, reaching about 20 to 40 per plant.37,38 The tubers continue to grow but not in number until the leaves dry. During the winter, the stolons decompose slowly, leaving the tubers of the plant separated.37
Current research on H. tuberosus is fundamentally based on the study of different varieties, different crop management techniques, plant selection and improvement, optimization of analytical determinations of sugars, etc., with the aim of obtaining higher yields and better inulin contents,22,30,37–41 obtained transgenic potatoes by transferring the genes (Ht1-SST and Ht1-FFT) isolated from H. tuberosus and expressed in potatoes, for which these could potentially be used as a nutritional supplement with properties that promote growth. health and/or as a crop capable of withstanding unfavorable conditions and having resistance against abiotic stress due to cold and drought fundamentally.
Yacon [Smallanthus sonchifolius (Poeppig & Endlicher) H. Robinson]
Like the topinambur (H. tuberosus), the yacón (Smallanthus sonchifolius) belongs to the botanical family Asteraceae (Table 2). It is a tuber plant native to the Andes,42 where it has been cultivated for a long time. Centuries by pre-Inca cultures whose original habitat is the highlands of the Andes, from southern Colombia to northern Argentina.43
|
Kingdom |
Plantae |
|
División |
Magnoliophyta |
|
Class |
Magnoliopsida |
|
Order |
Asterales |
|
Family |
Asteraceae |
|
Subfamily |
Asteroideae |
|
Tribe |
Smallanthus |
|
Species |
Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson |
|
SYNONYM |
Polymnia sonchifolia Poepp. & Endl. |
Table 2 Taxonomy of Smallanthus sonchifolius
Fountain: https://www.tropicos.org/name/2734075
Its name comes from the Quechua word Q.I. o Waywash, yakun (← yakunyuq), and substantive inflection of the yaku voice that names water, precisely the yacón is quite juicy and sweet. It acquires several common names by region or synonyms, such as llacón, jicama, Yacuma, puche, arizona, arikoachira, aricoma and racón.
Yacón consumption has been associated with the prevention of chronic diseases, such as: dyslipidemia and insulin resistance, as well as colon cancer, since it reconstitutes the beneficial microflora of the colon, improves calcium assimilation, corrects constipation, reduces blood cholesterol e for prebiotic, antidiabetic, antioxidant, and antimicrobial effects.44–48 It is an ideal food for diabetics and for people who want to lose weight since its consumption does not raise the concentration of glucose in the blood and provides very few calories to the diet. These properties are strongly associated with phenolic compounds and fructooligosaccharides (FOS) and it is proven that these compounds have beneficial effects on nutrition and health.49 In contrast to other root crops, which store carbohydrates in the form of starch, yacón accumulates carbohydrates such as inulin and fructooligosaccharides (FOS), fructose polymers, which cannot be hydrolyzed by the human body and pass through the digestive tract without Being metabolized, it does not increase the blood glucose level, providing calories lower than sucrose, excellent for hypocaloric diets and diets for diabetics.50–52 Yacón tubers have a pleasant, sweet taste.
Its importance lies in the presence of bioactive components present mainly in the tuber and leaves of the plant, which has aroused great interest due to its content of FOS and phenolic compounds with beneficial properties for health, since the low digestibility of FOS allows it to be consumed by diabetics, because they do not raise the level of glucose in the blood and their consumption is associated with other properties such as the reduction of cholesterol and triglycerides; improves calcium absorption, strengthens the immune system, prevents and reduces the risk of colon cancer, prevents constipation, and restores the intestinal flora,43,53–55 Yacón contains FOS (50–70% of its dry weight) and is therefore considered a prebiotic.) Yacón was found to prevent enteric infection caused by a strain of Salmonella enteritidis serovar Typhimurium (S. typhimurium) in mice.56 While in experiments carried out by Grancieri et al.,57 they observed that yacón flour supplemented in the diet of mice promoted beneficial effects on the health of the intestine of animals with induced colorectal cancer.
Phytochemical studies demonstrated the presence of bioactive sesquiterpene lactones in yacon, which induce different mechanisms of cell death in three cancer cell lines. The results indicate that yacón sesquiterpene lactones possess remarkable cytotoxicity, especially fluctuanine and polymatin, and may have the potential to be developed into therapeutically useful drugs,48,58,59 studied the chemical components of yacón leaves grown in China, determined the presence of antidiabetic components, monoterpenes, sesquiterpenes and diterpenes, which have a physiological role in antimicrobial activities and pest resistance, in addition to providing chemotaxonomic information. Meanwhile, research carried out in Japan managed to isolate substances such as uvedafolin, the leading compound of new anticancer agents, from yacón leaves.60 Due to its properties, research was carried out to study yacón syrup, produced from tubers, and to determine the feasibility of manufacturing candies and other products based on it.43,55,61
Due to the high concentration of fructans contained in the tubers of S. sonchifolius, the protective effects of its intake against colon carcinogenesis are investigated.62 De Moura et al.,32 determined the potential benefit of yacón intake for the prevention of colon cancer; their findings indicate that yacón can reduce the development of colon cancer. While the consumption of S. sonchifolius tubers is becoming more popular in the Japanese diet due to its low caloric value and high fiber content. Recent studies have suggested that feeding yacón prevents and controls diabetes by lowering blood glucose.50,55,64 However, in Peru, yacón is a traditional crop and is part of the food and medicinal biodiversity for its antioxidant, hypoglycemic and antibacterial properties. Due to these characteristics, its use as a functional food and/or nutraceutical is popular and promising.49,65 Both the roots and the leaves are used there, and it is marketed fresh and in the form of juices, syrups, tea in boxes with filter sachets.
Yacón-based beverages were shown to improve diabetic status in rats. Yacón has been widely recognized as an excellent source of bioactive compounds, including prebiotics and antioxidants,66–68 which may be beneficial to health. Experimental data indicate that prebiotics and some specific polyphenols could reduce the severity or incidence of degenerative diseases, such as diabetes. Dionisio et al.,69 evaluated the hypoglycemic effect of a beverage composed of yacón and Anacardium occidentale in diabetic rats induced by alloxan. The results strongly support that yacón and cashew-apple have important hypoglycemic properties that could improve diabetic status.
S. sonchifolius, native to the Andean region, grows between 2,000 and 3,500 meters above sea level. Its cultivation dates to pre-Columbian times and has remained for a long time restricted to very small areas since it is adapted to the ecological conditions of the Andes.1 Currently, distributed in much of the Andean territory as a wild plant or in cultivation, from northern Ecuador to northwestern Argentina. The center of diversity is located between the Apurimac basin in southern Peru (14° S) and La Paz in Bolivia (17° S), in this territory the highest genetic diversity of yacón is found.70 S. sonchifolius has been successfully cultivated in various regions and with different climates, including Brazil, Czech Republic, China, Korea, Japan, New Zealand, Russia, Taiwan, United States and Argentina.43,71–73
Botanical description: Smallanthus sonchifolius is an herbaceous and perennial plant, but it is cultivated as an annual, from 1.5 to 3 meters high.70 The stem is composed of a perennial underground part with annual aerial shoots that dry up after flowering. The aerial stem is cylindrical to angular, furrowed, hollow at maturity, and densely pubescent above. The leaves are opposite, with a decurrent blade towards the petiole; the leaf blade is broadly ovate with the base horned, auriculate or connate; the upper leaves are ovate-lanceolate; the upper part of the leaf is hairy and the lower or abaxial pubescent. Inflorescences in terminal capitula yellow to orange, with 1-5 axes, each with three capitula and densely pubescent peduncles (Figure 2). Each chapter has an involucre made up of 5 to 8 uniseriate and ovate filariae and two types of flowers: the radial ones that are ligulate and the disc ones that are tubular. The ligulate flowers, around 15, are female and the tubular ones are male. The fruit is an achene with ellipsoidal shape, dark brown color, with smooth epidermis, solid endocarp characterized by the free detachment of the pericarp with a light rubbing; some ecotypes do not produce fruits and if they do, they are not viable.74
The underground system consists of three parts: the rhizomes or adventitious roots, fibrous or thin roots, and storage or tuberous roots. The rhizomes are rich in fibers and give rise to new plants. The fibrous roots are very thin, and their function is the absorption of water and nutrients. The roots of reserves of up to 20 fleshy and tuberous storage roots.53 Storage roots form from a branching system of underground axes; they are mostly napiform, can reach up to 25 cm long and 10 cm thick and weigh between 0.2-2 kg.53 The color of the root bark and storage tissue varies, depending on the clone, from white, cream, pink (striated), lilac and even brown.75
The plant forms, between the stems and the roots, an irregular mass of reserve tissue, with many buds that give rise to shoots and is called "vine" or "crown" that is obtained after harvesting. From this organ, the traditional “seed” is obtained in the form of portions of the crown that are the propagules for planting or planting the crop (Figure 2). Therefore, the propagation of yacón for production purposes is predominantly vegetative, through propagules (or portions of the crown) with 4 or more buds or shoots.76,77
Yacón storage roots accumulate a large amount of inulin-type fructo-oligosaccharides (FOS). FOS constitutes about 10% of the fresh weight, and 70 to 80% of the dry weight. During storage of yacon storage roots, the concentration of FOS decreases. In New Zealand storage roots are stored for 30 days at 1°C with no change in FOS concentration. For the elaboration of products with a high concentration of FOS, the storage roots should be kept only for short periods in cold and dark spaces with high air humidity, and be processed, if possible, directly. Traditionally the storage roots remain in the sun for a couple of days after harvest, as this way the roots become sweeter due to the 40% loss of water. This means that the absolute amount of FOS is higher directly after harvest and the relative amount is higher after sun exposure. Storage conditions must be selected depending on the type of product desired.70
The tubers are consumed as a fresh fruit and used for making juices and meals. While the processed tubers, such as dehydrated flakes, jelly, sweet, pickled, jam, juices, liquor and with tea leaves. In addition, the whole plant is used as fodder and the stems are used to make "yista", "llicta" or "llipta" (a solid preparation made with the ashes of these or other plants and used in the coking process). or coca insalivation).78,79
The main purpose of this review was to know and determine the scientific characteristics of little-known crops such as topinambur (H. tuberosus) and yacón (S. sonchifolius), for which the completion of this work required the search with support scientist of numerous articles that report on the growth, development and production of topinambur and yacón crops. It was possible to establish the antecedents of the topinambur and yacón species that are linked to their morphology, composition, production, different uses, industrialization and fundamentally on their use in human nutrition, given by the effect of the consumption of these crops on the nutrition and health.
This work contributes to the dissemination of scientific knowledge of topinambur (Helianthus tuberosus L.) and yacón [Smallanthus sonchifolius (Poeppig & Endlicher) H. Robinson] crops. For all the above, it is observed that these crops have common characteristics, in addition to belonging to the same botanical family, they are ancestral crops produced for their different uses (horticultural, forage and industrial), for generating tubers that store carbohydrates such as inulin and fructooligosaccharides , and the consumption of these tubers is associated with the prevention of some diseases and they are considered an ideal food for diabetics and people who have obesity problems.
Therefore, both are multipurpose and nutraceutical crops with great potential, because they provide food and provide health benefits.
None.
The authors declare no conflict of interest.
None.

©2022 Gabriela, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.