Journal of
eISSN: 2572-8466


Review Article Volume 6 Issue 2
Departament of Natural Products, FIOCRUZ, Rio de Janeiro, Brazil
Correspondence: Raquel Elisa Silva-López, Departament of Natural Products, Oswaldo Cruz Foundation (FIOCRUZ), Av. 4365, Rio de Janeiro, RJ, Brazil
Received: March 07, 2019 | Published: March 28, 2019
Citation: Silva-López RE, Gonçalves RN. Therapeutic proteases from plants: biopharmaceuticals with multiple applications. J Appl Biotechnol Bioeng. 2019;6(2):101-109. DOI: 10.15406/jabb.2019.06.00180
Proteases represent a significant group of industrial enzymes, and a growing class of therapeutic agents. They catalyze the cleavage of peptide bonds and are found in all living organisms. Plant proteases have expressive stability with good enzymatic activity, and have been investigated for therapeutic purposes. Their extraction is simple with low cost and their preparations have no pathogenic potential for humans or animals. Nowadays, they are used in the treatment of wounds, cancer, infectious diseases and digestive disorders. This review will address some chemical, biological and pharmacological aspects of the most important therapeutic plant proteases: papain, bromelain, ficin and cucumisin. Papain, extracted from latex of papaya (Carica papaya) and bromelain from fruit and stem of the pineapple (Bromeliaceae family), are Cysteine proteases andhave broad medicinal applications. Ficin, a general designation for cysteine proteases obtained from latex of fig tree (Ficusspp.), has been traditionally used as a vermifuge. Cucumisin, is the only serine protease, was first isolated from melon fruits (Cucumis melo). However, other cucumisin-like serine proteases have been found in plants from different botanical families. Although they are well-characterized proteases, their medicinal and biotechnological potentials are not extensively explored.
Keywords: Therapeutic proteases, plants, papain, bromelain, ficin, cucumisin
Human beings have used plants to treat diseases and adverse conditions since ancient times, and consequently, employed their proteases empirically.1 Nowadays, proteases represent a very significant group of industrial enzymes, accounting for 60% of the total enzyme market, especially the proteases from microorganisms due to ease of modifying their biochemical characteristics through genetic mutations.2 In addition, plant proteases are part of a growing class of therapeutic agents because many efforts have been made to exploit biodiversity.3 The Table 1 summarize some application of general proteases in different kinds of industry. 4–6
Protease |
Source |
Application |
Actinidin |
Kiwifruit |
Healing of neuropathic diabetic foot ulcer, increase protein digestion and ameliorate of constipation in animals/ Dietary supplement and meat tenderizing. |
Bromelain |
Pineapple |
Treatment of thrombosis, rheumatoid arthritis, wounds, cancer, asthma, angina, bronchitis, sinusitis, osteoarthritis, surgical traumas, pyelonephritis, mal absorption of drugs, alleviates pain and swelling, disorders of the blood vessels and heart, inflammatory diseases in general/ Meat tenderizing, ethanol production, baking, textile, cosmetic and animal feed industriesy, antibrowning, tooth withening, fish protein hydrolysis. |
Cardosin |
Cardoon |
No therapeutic effect investigated/ Milk clotting and manufacturing of cheese. |
Cucumisin |
Melon |
Fibrin clotting lysis in thrombotic disorders / Meat tenderization, collagen hydrolysis to obtain gelatin, milk clotting, enzyme-catalyzed synthesis of dipeptides. |
Ficin |
Fig |
Vermifuge/ Synthesis of bioactive peptides and immunoglobulin G cleavage. |
Oryzasin |
Rice |
No therapeutic effect investigated/ Milk clotting. |
Papain |
Papaya |
Treatment of edemas, sinusitis, gluten intolerance, hypochlorhydria, digestive disorders, caries removal, healing burn wound, infections, cancer/ Detergent, leather treatment, meat tenderizing, enzymatic bioactive peptide synthesis, antibody fragment production, proteomic applications, chill profile, removal of haze in beverages, modification of protein rich material to reduce allergy. |
Phytesin |
Barley |
No therapeutic effect investigated/ Milk clotting. |
Zingipain |
Zinger |
Anti-proliferative agent in cancer animal model/ No biotechnological uses investigated. |
Table 1 Examples of plant proteases and therapeutic/biotechnological applications 4–6
Proteases catalyze the cleavage of peptide bounds in peptides and proteins, mainly by hydrolysis or ammonia elimination, resulting in peptides with variable sizes and free amino acids. They are found in all living organisms, tissues, cells and organelles and are encoded by approximately 2% of the genes of an organism.7 Proteases mediate and/or participate in various physiological processes including: protein turnover to provide a source of amino acids or of carbon and nitrogen containing intermediates necessary for the synthesis of new molecules and proteins. They are also involved in the digestion of food proteins; tissue remodeling; processing of neuropeptides, hormones and proenzymes; intracellular proteolysis by proteasomes and lysosomes; blood clotting; complement activation cascade reactions; metabolism regulation and a vast number of other biological phenomena.8 These enzymes are able to catalyze reactions in different environments under varying conditions, and there are a many types of proteases. They can act near the ends or within polypeptide chains and are denominated exopeptidases and endopeptidases, respectively. They display distinct catalytic mechanisms, according to the presence of different amino acids in the active site, such as serine, cysteine, aspartic, threonine, glutamic, asparagine and metalloproteases.9
Plant proteases are found in all the botanical families and are important mediators of a striking variety of biological processes, since they cleave specific peptide bonds. Proteolysis controls gene expression that is responsible for cell growth, differentiation, developmental division or reproduction. It is also involved in senescence, meiosis, gametophyte survival, epidermal cell fate, stomata development, chloroplast biogenesis, removal of damaged or improperly folded proteins, processing and targeting of proteins, zymogens and peptide hormones through digestion of signal peptides. It participates in programmed cell death, and local and systemic defense responses.10 The most remarkable feature of these enzymes is the high capability for hydrolysis at higher temperatures than animal enzymes. Due to the expressive thermal stability associated with good protease activity, plant proteases have been investigated for therapeutic purposes.11,12
Therapeutic proteases
Enzymes are the most important targets in rational drug development but may also have therapeutic applications in certain diseases and conditions and are then denominated as therapeutic enzymes. So, therapeutic proteases are enzymes used in the treatment and the diagnosis of diseases and also in surgical procedures.3 At the end of the last century, proteases were initially used as therapeutic agents in haemostasis disorders such as coagulation factors VIII and IX to treat respectively, haemophilies A and B, and urokinase, which converts plasminogen into plasmin, which in turn degrades fibrin polymers, being used in cases of thrombosis. These enzymes were obtained from natural sources, human and animal plasma, but large amounts of material were required to obtain a small amount of these serine proteases. In addition to the low yield of these enzymes, there was the imminent risk of contamination of the source material with pathogenic infectious agents which could cause secondary infections and immunological reactions in patients who received them.13 Nowadays, they are produced by recombinant DNA technology, where genes encoding proteases of interest are sequenced, cloned, inserted into suitable expression vectors and overexpressed in specialized microorganisms chosen according to the complexity of the protein. So, proteases are purified, standardized and finally formulated for human or veterinary use. Although it is a complex and expensive procedure, it guarantees quality and the absence of pathogenic infectious agents.14
In general, therapeutic plant proteases are not heterologous proteins obtained directly from the organs of the plant or from its latex, and it is possible to extract the protein without affecting the viability of the plant. Their preparations have no pathogenic potential for animals, since they do not carry pathogenic infectious agents. In addition, plant proteases are simple and relatively easy to obtain at low cost of production.15 They are widely used as therapeutic enzymes in the treatment of wound healing, cancer, digestive disorders, infectious diseases and in various processes of the food industry such as brewing, meat tenderizing and milk-clotting.16–18 Some chemical, biological and pharmacological aspects of the most studied plant proteases papain, bromelain, ficin and cucumisin will be described.
Papain
Papain (EC 3.4.22.2) is a cysteine protease extracted from the latex of Carica papaya, a tropical fruit known as papaya. The activity of this enzyme is related to the fruit maturation: the greener the fruits, the greater the activity. It was the second protein to be crystallized (1968) and the first cysteine protease with the three-dimensional structure elucidated (1984). It is also a model of the papain-like cysteine protease family and helps in the understanding the mechanism of action of cysteine proteases.19,20 It is an endopeptidase that belongs to the papain superfamily and subfamily C1A by MEROPS database.7 It is a zymogen with a 345 amino acids (inactive form) and after the removal of the 133 amino acids N-terminal region, it becomes active.21 Active papain is thus a single polypeptide chain with 212 aminoacids and 23,406 Da, showing all-α and antiparallel β-sheet domains, resulting in a three-dimensional globular structure stabilized by three disulfide bridges that contribute to the high stability of the enzyme. The activity is associated with the presence of catalytic residues Gln19, Cys25, His158, His159 and Trp177.22 The residues Cys25 and His158 make up part of the catalytic diad and the other aminoacids are responsible for the nucleophilic character of the active site.23
This cysteine protease is stable at high temperatures, elevated concentrations of organic solvents or denaturant agents in a wide range of pH (3.0-9.0) and, it has the maximum activity at pH 6.0-7.0 and 60°C.21 Papain activity is affected by cysteine protease inhibitors such as 4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride) (AEBSF), trans-epoxysuccinyl-L-leucyl- amido-(4-guanidino) butane, L-trans-3-carboxyoxiran-2-carbonyl-L-leucyl-agmatine, cystatins, N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide (E-64), serine protease inhibitors as phenylmethanesulfonyl fluoride (PMSF), 𝑁-tosyl-L-leucylchloromethyl (TLCK), 𝑁-tosyl-L-phenylalanine chloromethyl (TPCK), α2-macroglobulin and other inhibitors such as antipain, leupeptin, alkylating and sulfhydryl binding agents, carbonyl reagents and heavy metals.24 It has a broad selectivity towards many proteins and peptides, particularly substrates that containing basic amino acids (Arg and Lys) and residues following Phe (Figure 1).25
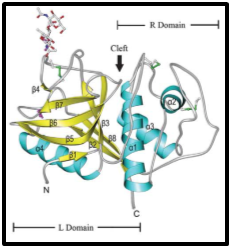
Figure 1Ribbon representations of the molecular structure of papain-like peptidases. The polyethylene glycol molecule is represented by a stick model.23
Other cysteine proteases are also found in papaya fruit latex, such as chymopapain (EC 3.4.22.6), glycyl endopeptidase (EC 3.4.22.25) and caricain (EC 3.4.22.30). Papain is a minor constituent (about 5–8%) among the protein constituents,26 however, this enzyme is widely studied due to the high activity, important stability and, its easy purification by ion exchange or affinity chromatography.20 Papain is mainly used in industry in pharmaceutical preparations, detergent formulation, leather treatment, meat tenderizing and beer clarification because the strong activity towards proteins, peptides and substrates containing amino acids linked by ester and amide bonds.19 The enzyme has broad medicinal uses in the treatment of edemas, allergic sinusitis, leaky gut syndrome, gluten intolerance, hypochlorhydria and other digestive disorders as well as in the removal of caries.22 In addition, papain has antibacterial activity against Alicyclo bacillus, Bacillus subtilis, Enterobacter cloacae, Escherichia coli, Listeria monocytogenes, Salmonella typhimurium, Staphylococcus aureus and Proteus vulgaris.27 Antihelmintic action has been observed in vitro and in vivo against Ascaris suum, Haemonchus contortus, Heligmosomoides polygyrus, Trichuris muris, Protospirura muricola and Strongyloides venezuelensis.28–31 Antifungal activity has been demonstrated toward Aspergillus niger, Candida albicans, Mucor spp. and Rhizopus spp., where the possible mechanism of action may be the cleavage of the protein content of glycoproteins in the cell membrane.32
This protease has a strong anti-angiogenic effect in vitro using endothelial cells from human umbilical cord as a model. In low concentration, a distinct inhibitory effect is exerted on cell growth, cell migration and tube formation and this effect may be due to interference with protein kinase B (AKT), mitogen-activated protein kinase kinase (MEK) 1/2 and Stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) phosphorylation levels.33 Associated with bromelain, papain demonstrated antitumor activity, inhibiting NFκB/AMPK and downstream signalling proteins such as phosphorylated-AKT, phosphorylated extracellular signal-regulated kinases (ERK), phosphorylated-signal transducers and activators of transcription 3 (Stat3), that affect proliferation, invasion and migration of the tumor. It also induced apoptosis in intra- and extra-hepatic human cholangiocarcinoma cell lines.34
Papain was also employed in tissue debridement and to stimulate the wound healing process, in cases such as pressure ulcers, diabetic, leprosy and chemotherapeutic lesions, Fournier's syndrome, and other injuries, through the proteolysis of necrotic tissues and cell fragments, helping joining of wound edges and accelerating tissue regeneration. Furthermore, papain reduces the pH of wound stimulating the production of cytokines that promote cell repair that, and decreasing the growth of microorganisms and inflammatory responses.16 A novel elastic liposome containing papain was an excellent topical formulation for hypertrophic scar treatment. The scar elevation index, microvascular density, and collagen fiber were significantly decreased with regular arrangement. The expressions of tissue growth factor-β1, and nuclear factor kappa B-p65, which play a crucial role in inflammatory and immune responses in hypertrophic scar were significantly down regulated in contrast with those in a control group.35
Excavation of carious tooth tissues is a restorative approach that eliminates infected tissue, controls lesion progression, and removes necrotic, softened dentine, to allow proper support for the filling. Gels based on papain have antibacterial and anti-inflammatory activities and constitute a non-invasive technique for caries removal. Papain eliminates infected dentine withdrawal debris, with no harmful effect on non-damaged tissues, breaks down degraded collagen fibrils and constitutes an alternative for caries treatment.36 In plant physiology, papain is a defence mechanism of wounds in the C. papaya plant. The enzyme leads to latex coagulation, forming a physical barrier that is a primary step in the defence mechanism, playing crucial roles in plant-pathogen interactions.37
Papain is the best studied cysteine protease, however it is not appropriately exploited as a therapeutic protease. There are few studies about the therapeutic applications and no reports in relation to the toxicological data, although affords a model of study for similar cysteine proteases which are important therapeutic agents for many diseases.19–22,33–35
Bromelain
Bromelain is a crude aqueous extract rich in cysteine proteases obtained from the stem and fruit of plants of the Bromeliaceae family, of which pineapple, (Ananas comosus, syns. A. sativus, Ananassa sativa, Bromelia ananas), is the best known species. In this extract there are non-proteolytic constituents, such as escharase, glycosidases, peroxidases, ribonucleases, cellulases, carbohydrates, protease inhibitors (PIs) and other substances. Pineapple tissues contain at least four glycosylated proteases with molar masses from 20 to 31 kDa and isoelectric points between 4.6 and 10 that belong to the papain superfamily: stem bromelain (EC 3.4.22.32), fruit bromelain (EC 3.4.22.33), ananain (EC 3.4.22.31) and comosain. These enzymes together have the protease activity of bromelain and show expressive stability at high temperatures. The amino acid sequence of bromelain has a close similarity to that of papain, caricain, actinidin, and chymopapain.38
Stem bromelain has pI of 9.5, and is the most abundant protease in pineapple tissue preparations. It consists of a single polypeptide chain with 212 amino acids linked to an oligosaccharide chain, and a molar mass around 23.8 kDa. In terms of specificity for hydrolysis of peptide bonds, ananain and fruit bromelain are typical members of the papain family, possessing a preference for hydrophobic residues in P2. Stem bromelain is atypical because it requires an arginine, both at P1 and P2 in small substrates, and maximal activity at a pH range of 5.5–8.0. On the other hand, fruit bromelain has a pI of 4.6 and is an acidic protein.39
Bromelain is prepared from pineapple juice by centrifugation, ultrafiltration, and lyophilization that yields a yellowish powder and the protease activity is determined with casein, gelatin or chromogenic substrates.17 It has been employed in many types of industries such as food, beverage, tenderization, cosmetic, textile and pharmaceutical (Figure 2).5
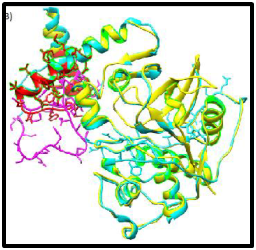
Figure 2 Ribbon representations of the molecular structure of bromelain-procaricain predicted by modeller. The superimposed between bromelain -procaricain model (cyan) and procaricain template (yellow). The purple and red sticks representation refer to the side chains of unfavourable region.40
Pineapple (chemically known since 1876) has been used as a medicinal plant in several native cultures and their medicinal properties are attributed to bromelain.1 Due to the complex composition, it interacts with a great variety of molecules and is responsible for a diversity of pharmacological effects. Thus, it has been used for the treatment of many diseases and conditions such as rheumatoid arthritis, thrombophlebitis, wounds, cancer, angina, bronchitis, sinusitis, osteoarthritis, surgical traumas, pyelonephritis, and for enhancing the absorption of certain drugs. In all cases, this extract significantly alleviates pain and swelling, and decreasing the healing time required with conventional treatments.38,39-41
Bromelain reduces the risk of arterial thrombosis, embolism and disorders of the blood vessels and heart, such as heart attacks, stroke, hypertension and heart failure.42 These effects are due to the inhibition of cyclooxygenase-2 (COX-2), that reduces the level of prostaglandin E2 (PGE2) and thromboxane A2, and increases prostacyclin, which inhibits blood platelet aggregation and increases the fibrinolytic activity that prevents the clot formation. Bradykinin generation at an inflammatory site is also reduced via depletion of the kallikrein system, and the formation of fibrin is limited, reducing plasma prekallikrein, high molecular weight kininogen and thrombin as well as hydrolysis of cholesterol plaques, that minimize the clot formation and blood vessel blockade.43
Intestinal infections by Vibrio cholera and Escherichia coli, whose enterotoxins are structurally related and cause waterborne acute diarrhea in animals, are strongly affected by oral treatment with bromelain. It acts as an anti-adhesion agent by proteolysis the glycoprotein receptor located on the intestinal mucosa attachment sites and activates receptors that regulate the intestinal secretory signaling pathways of cyclic guanosine monophosphatase and calcium-dependence. In vitro evidence also suggests that bromelain exerts anti-helminthic activity against the gastrointestinal nematodes, Trichuris muris and Heligmosomoides polygyrus and acts as an anti-fungal agent by stimulating phagocytosis and respiratory burst killing of Candida albicans.38,42
Treatment using bromelain attenuates the development of asthma in a murine model, and may have similar effects in the treatment of human asthma and hypersensitivity disorders. It showed important anti-inflammatory activity, due to the reduction of the number of total leukocytes, eosinophils, and cellular infiltrates via lung pathology, as compared to the control group. Besides this, the extract significantly reduced cluster of differentiation (CD) 4+ and CD8+ T cells without affecting cell numbers in the spleen or hilar lymph node and, decreased interleukins (IL) IL-4, IL-12, IL-17, as well as interferon (IFN) α in the serum of bromelain-treated animals.44
Bromelain interferes in cancer development during cellular transformation, proliferation, angiogenesis, invasion and metastasis and this activity is due to the direct impact on cancer cells and their microenvironment in many ways. COX-2 is a crucial component of cancer-associated inflammation that is involved in the synthesis of PGE2, which acts as a promoter of tumor angiogenesis and progression. Bromelain inhibits COX-2 activity and, consequently, decreases PGE2 levels in murine and human cancer cell lines. The extract also activates the inflammatory mediators IL-1β, IL-6, INF-γ and tumor necrosis factor (TNF)-α in mouse macrophages and human peripheral blood mononuclear cells, indicating that bromelain activates the healthy immune system. Conversely, it reduces IL-1β, IL-6 and TNF-α secretion when immune cells are already stimulated. The cell surface marker, CD44 is expressed by cancer and immune cells directly involved in cancer growth and metastasis and regulates lymphocyte requirement at the site of inflammation. This extract rich in proteases reduces the level of CD44 expression on the surface of mouse and human tumor cells, and regulates lymphocyte homing and migration to the sites of inflammation. Besides, bromelain activates natural killer (NK) cells, augments the production of granulocyte-macrophage-colony stimulating factors IL-2, IL-6 and, decreases the activation of T-helper cells, increasing the tumoricidal capability of host immune system. It selectively induces apoptosis in tumor cells due to the increment of p53 and Bax expression, the activators of apoptosis. Besides, it decreases the activity of cell survival regulators, Akt and Erk, promoting apoptotic cell death in tumors.38,42,43–45
Bromelain decreases inflammatory mediators and is anti‑inflammatory agent in various conditions. It has been employed as adjuvant therapy in the treatment of chronic inflammatory and autoimmune diseases, such as rheumatoid arthritis and osteoarthritis, since it can modulate surface adhesion molecules on T cells, macrophages, NK cells and induces secretion of IL-1β, IL-6, TNF-α. Besides, it has analgesic properties since it decreases the levels of pain mediators PGE2 and bradykinin.42,45,46
Debridement is the procedure of removing dead, infected, senescent, and/or devitalized tissues, since these tissues interfere in the healing of a wound. Debridement transforms a chronic to an acute wound and, reduces the bacterial burden. Bromelain proteases degrade necrotic debris and regulate cell maturation and multiplication, collagen synthesis, development and removal of perivascular fibrin. They also hydrolyze collagen, elastin, laminin, fibronectin and other damaged components of the extracellular matrix, releasing growth and angiogenic factors sequestered in this matrix, activating bioactive chemokines and cytokines.47,48 In this way the extract promotes the effective healing of wounds. Topical formulations (35% bromelain) also contains escarase that efficiently removes eschars. Debridement accelerated recovery of blood perfusion, controlled the expression of TNF-α, which provide a chemotactic gradient for additional leukocytes to enhance the inflammatory process, stimulates cytokine secretion and enhances fibroblast and smooth muscle cell chemotaxis, reprogramming the wound microenvironment.49,50 Bromelain debridement is better than surgical debridement, because surgical incision is painful, non-selective and exposes the patients to the risk of repeated anaesthesia, and bleeding, reduces the time to complete wound healing, and reduces morbidity and mortality of severely burned patients and problems of infection.51,52
Bromelain has very low toxicity with an LD50 greater than 10 g/kg in mice, rats, and rabbits. Toxicity tests on dogs, with 750mg/kg/day of bromelain, showed no toxic effects after six months. Dosages of 1,5g/kg/day when administered to rats showed no carcinogenic or teratogenic effects and did not provoke any alteration in food intake, histology of heart, growth, spleen, kidney, or hematological parameters. In a rat study, it was absorbed intact through the intestine, with up to 40% of high molecular substances detected in the blood after oral administration. This extract retained the protease activity in plasma and formed a complex with α2-macroglobulin and α1antitrypsin the most important PIs of plasma.53
Ficin and ficin-like proteases
The Ficus genus belongs to the Moraceae family and the common characteristic of all species is the presence of latex within laticiferous cells. Latex is a complex, sticky fluid, milky in appearance, excreted in response to injury to protect the plant from pathogen invasion as mentioned for papain. Proteolytic fractions of Ficus latices predominantly contain cysteine proteases, but there are also aspartic and serine proteases.54 The latex from the fig tree, Ficus carica, constitutes an important source of many protease components, known under the general term ficin (EC 3.4.22.3), which belong to the cysteine proteases of the papain family (family C1, clan CA).55 Ficin was first crystallized by Walti in 1938 and is composed of glycosylated proteases with high protease activity.56 Many ficins with distinctive molecular structures and catalytic properties have been identified.57 Six ficin isoforms A, B, C, D1, D2 and E from F. carica are well characterized. They are single polypeptide chain basic proteases with molecular mass about 24 kDa and the N-terminal sequence has homology with the N-terminal sequences of other plant cysteine proteases. The maximal activity of ficin is in the pH range of 6.5-8.5 and the enzymes have preferences for basic residues at the P1 position, while for dipeptide substrates, an aromatic residue at the P2 position enhanced the catalytic efficiency.56 This protease has intrinsic peroxidase-like activity at an active site different from that of protease activity, which shows that this enzyme is able to catalyze more than one kind of reaction, and thus support a wider application for biotechnology purposes (Figure 3).58
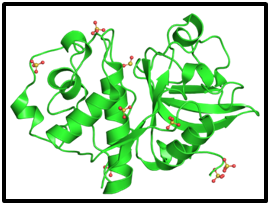
Figure 3Ribbon representations of the molecular structure of ficin (green). Solvent molecules are represented by a stick model.
Ficin can be employed in many areas of the food industry such as in meat tenderization,59 collagen hydrolysis to obtain gelatin and, milk clotting60 to the high caseinolytic activity.54 It has also biotechnological importance since it has been used as a biocatalyst for enzyme-catalyzed synthesis of dipeptides61,62 and when compared to other cysteine proteases like papain and bromelain, ficin was shown to be more specific for mouse IgG1 cleavage, giving better yields and better immunoreactivity.63
The partially purified ficins showed pro-coagulant activity through activation of the human coagulation factor X.64 The latex of some species of Ficus has been traditionally used as vermifuge in Central and South America. Capsules containing F. glabrata latex have been commercially exploited due to the high ficin content that digest live intestinal worms. Pharmacological studies with live Ascaris demonstrated a lethal effect at 0.05% latex in physiological saline solution. A clinical trial with 181 persons has resulted in a recommended dosage of 1.0cm3 of prepared latex/kg/day for 3 days to be repeated every 3 months without gastrointestinal toxicity.65 Anthelmintic activity of latex from F. insipida Willd and F. carica was investigated in mice naturally infected with Syphacia obvelata, Aspiculuris tetraptera and Vampirolepis nana. The lattices were administered by the intragastric route in doses of 4 ml/kg/day during three consecutive days and was effective in the removal S. obvelata, but not for other helminthes.66
Although ficin is employed in many biotechnological processes in the food industry,55–59 its only known pharmacological use is to kill intestinal worms.65,66 However, no toxicological and clinical trials concerning to ficin were conducted.
Cucumisin and cucumisin-like proteases
The fruits of some Cucurubitaceae species contain significant amounts of serine protease activity. An extracellular thermostable alkaline serine protease isolated from the sarcocarp of melon fruit, Cucumis melo L. var. Prince called cucumisin (EC 3.4.21.25) was the first characterized plant subtilisin class protease.67 It comprised more than 10% of the total juice protein, suggesting an important function during fruit development.68 This enzyme was isolated in another variety of C. melo69–71 and other species of the Cucumis genus such as in C. trigonus Roxburghi.72 Cucumisin-like serine proteases were also found in organs from plants other than those of the Cucurbitaceae family such as in F. religiosa73 Solanum elaeagnifolium,74 Hordeum vulgare L.,75 Lycopersicon esculentum,76,77 Morus indica,78 Faseolus vulgaris,79 Euphorbia supine,80 Alnus glutinosa and Casuarina glauca,81 Taraxacum officinale Webb S. L.82 Benincasa hispida,69 Maclura pomifera (Raf.) Schneid,83 Trichosanthes bracteata and Trichosanthes cucumeroides Maxim.84 In Arabidopsis, 56 genes predicted to encode subtilases have been annotated. Plant subtilisin-like serine protease (subtilases) are involved in many physiological processes, such as microsporogenesis, symbiosis, hypersensitive response, signal transduction and differentiation, senescence, and protein processing.85
These enzymes are alkaline serine proteases with pH optima around 10, an optimal temperature of about 70°C, with specificity for Leu, Asn, Gln, Thr and especially Met at the P1site when using synthetic oligopeptides. This protease tended to avoid charged aminoacids (Asp, Glu, Lys and Arg) and did not cleave Gly, Ile or Pro at the P1.86 Cucumisin is classified into the subtilisin family, because the amino acid residues of the catalytic triad are characterized by Asp140, His204 and Ser525 residues that are identical to the triad from Bacillus subtilisins in terms of the primary structure. In addition, the amino acid sequence Gly-Thr-Ser-Met around the reactive serine residue of cucumisin is identical to that of subtilisin, a Bacillus subtilis serine protease called subtilase.87 It is an endopeptidase that belongs to family S8 and clan SB which are subdivided into six families based on their sequence similarities. Most plant subtilases are grouped into the pyrolysin family, which is characterized by a large insertion between the stabilizing Asn and the reactive Ser and/or long COOH-terminal extensions.82 Some of them are expected to have a broad substrate range, others are considered to be specific prohormone convertases (Figure 4).88
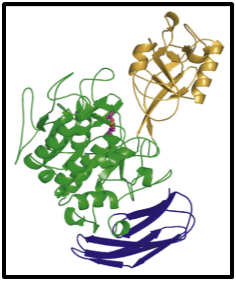
Figure 4Ribbon representations of the molecular structure of monomer cucumisin. The catalytic, protease-associated, and fibronectin‐III-like domains are colored green, orange, and blue, respectively. Diisopropyl fluorophosphate is depicted by a stick model in red.69
A native form of cucumisin from the juice of developing melons has a molecular mass of 67 kDa with 621 residues and is synthesized as a proenzyme. The 2.5kDa of signal peptide (pre-sequence, 22 residues) is removed from cucumisin precursor in the endoplasmic reticulum and the 10kDa of the N-terminal propeptide (88 residues) is autocatalytically removed to produce 67kDa of mature cucumisin.89 The crystal structure of mature cucumisin is reported to be composed of three domains: the subtilisin-like catalytic domain, the protease-associated domain and the C-terminal fibronectin-III-like domain. A structural homology search indicated that the catalytic domain of cucumisin shares structural similarity with subtilisin and subtilisin-like fold enzymes.68,90
Until now cysteine proteases are extensively used in the food and medicine industry. However, their activities are affected by air oxidation or metal ions, and by reducing or chelating agents. In contrast, various serine proteases including plant proteases have no requirement for any co-factors.91 Cucumisin demonstrated to have important milk-clotting activity compared with other plant proteases such as papain and ficin, is more stable than papain.92 A cucumisin-like serine protease from the leaves of Solanum tuberosum (StSBTc-3) was able to degrade human fibrinogen and to produces fibrin clot lysis in a dose dependent manner. Additionally, this protease was able to inhibit platelet aggregation and did not demonstrate cytotoxic activity on human erythrocytes in vitro at all concentrations assayed, suggesting that StSBTc-3 could be evaluated as an agent in the treatment of thromboembolic disorders such as strokes, pulmonary embolism and deep vein thrombosis.93
Although cucumisins are so well-characterized proteases, there is only a report about the pharmacological effect of an enzyme from S. tuberosum.93 and no clinical or toxicological study reported. However, these enzymes are thermal stable and exhibited important protease activity, essential technical features for the employment as therapeutic proteases.
Proteases are essential in a great diversity of aspects of life and death from all plants.93 The most investigated therapeutically are papain, bromelain, ficin and cucumisin and this review summarizes some biochemical aspects and pharmacological applications from these enzymes. Plants are reliable sources of proteases with expressive activity and stability and easy obtainment.94 They are commercially important enzymes due to their multiple applications in the food, biotechnology, pharmaceutical and other types of industries, where conventional industrial processes can involve the breakdown of proteins.14 Plant extracts with a high content of proteases have been used in traditional medicine for a long time for the treatment of diverse diseases and conditions such as digestive disorders,13–15,19 wound debridement,46–49 inflammation and immune-modulation with excellent therapeutic indexes.35,38-42,45 Other plant proteases have been isolated, purified, characterized and their biological functions and pharmacological properties investigated and their therapeutic applications are under investigation.8,9
The authors are grateful to Dr. Benjamin Gilbert, senior researcher ofNatural Products Department at FIOCRUZ for paperrevision.
All authors declare no conflict of interest.

©2019 Silva-López, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.