Journal of
eISSN: 2572-8466


Research Article Volume 9 Issue 3
1Enviornmental Microbiology Laboratory, Research Institute of Chemistry and Biology, Mexico
2Department of Parasitology, Universidad Autónoma Agraria Antonio Narro, Saltillo, Coahuila, Mexico
Correspondence: Juan Manuel Sanchez-Yanez, Enviornmental Microbiology Laboratory, Research Institute of Chemistry and Biology, Ed-B3 C.U., Universidad Michoacana de San Nicolás de Hidalgo, Francisco J. Mujica S/N, Col. Felicitas del Rio C.P. 58000, Morelia, Mich, México
Received: April 24, 2022 | Published: May 23, 2022
Citation: González-Campos M, Marto-Domínguez G, Ignacio-De la Cruz JL, et al. Stenocereus queretaroensis a source of endophytic plant growth promoting bacteria for cropping Zea mays. J Appl Biotechnol Bioeng.2022;9(3):76-81. DOI: 10.15406/jabb.2022.09.00288
Healthy growth of Zea mays requires NH4NO3 as nitrogen fertilizer (NF), and its uptake is important to avoid loss of the NF. An alternative solution to enhance the root uptake capacity of Z. mays of NF at a dose to supply Z. mays demand without compromise its health; with beneficial entophytic genera and species of Stenocereus queretaroensis of the type Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus. The objectives of this research were: a) to select from the interior of roots of Stenocereus queretaroensis: B. vietnamiensis and G. diazotrophicus, b) to analyze the growth of Z. mays with B. vietnamiensis and G. diazotrophicus and NF at 50%. B. vietnamiensis and G. diazotrophicus were recovered from the roots of S. queretaroensis and inoculated on Z. mays seed with NF. Using the response variables: percentage of emergency, phenology and biomass to seedling and flowering, the experimental data were analyzed by ANOVA-Tukey (P ≤ 0.05). The percentage of emergency, phenology, and biomass at seedling and flowering of Z. mays with B. vietnamiensis and G. diazotrophicus at 50% of NH4NO3, registered numerical values with statistical difference compared to those obtained in Z. mays without B. vietnamiensis and G. diazotrophicus only with NF at 100% or relative control (RC). This supports that B. vietnamiensis and G. diazotrophicus, entophytes of S. queretaroensis, invading the interior of Z. mays roots, converted metabolites related to root physiology into phytohormones that allowed maximum root uptake of NH4NO3 at 50%.
Keywords: soil, xerophytic plant, domestic crop, chemical fertilization, endophytic bacteria
The healthy growth of Zea mays (maize) demands NH4NO3 as nitrogen fertilizer or NF.1,2 This NF normally is not uptake by root system at 100% dose, due to several problem related with complex soil-root-microorganisms system in contrast not absorbed NF could caused loss fertility, or surface and groundwater contamination.3 An ecological alternative solution to optimize reduced dose of the NF in Z. mays is to treat its seeds with: Bacillus subtilis,4 Paenibacillus polymixa,5 or Pseudomonas putida6 including Burkholderia cepacia,7 all of them are able to convert exudates organic compounds from the seed or/and the root into phytohormons,8,9 to enhance the growth of an extensive and dense root system for reduced dose of NF.10,11 In the literature it has been shown that some genera and species of those previously mentioned are specific for certain varieties of Z. mays, which can be relatively displaced by native soil rhizobacteria that colonize the roots of this cereal, without an evident beneficial effect on the uptake and optimization of NF reduced to 50% of what is recommended. An alternative solution to enhance and uptake NF at 50% is the selection of genera and species of endophytic bacteria that promote plant growth since they are competitive and effective in enhancing NF at 50%, without compromising healthy of Z. mays.12–14 This endophytic bacteria can be to recover from xerophytes such as Stenocereus queretaroensis called “pitayo” in Spanish;15 a cactus adapted to water stress due to low humidity, drastic changes in temperature; chemical related to the limitation of essential mineral salts of N (nitrogen), PO4-3 (phosphates) and K (potassium): which includes biological ones such as root phytopathogens, given that S. queretaroensis grows successfully without human protection in this environment.16,17 The hypothesis of this research is that certain endophytic genera and species such as Burkholderia vietnamiensis18,19 and Gluconacetobacter diazotrophicus20,21 recovered from inside of the roots of S. queretaroensis could be benefical for domestic crop such as Z. mays, in enhancing uptake of NF at 50% through B. vietnamiensis and G. diazotrophicus, by invading the root system of Z. mays, since they would avoid competition with other microorganisms in the rhizosphere and the soil, by living inside of root system to convert organic compounds into phytohormons,21–23 which maximize the radical uptake capacity in NF reduced at 50%, and even that healthy growth of Z. mays. Therefore, the objectives of this research were: a) to select from inside S. queretaroensis roots: B. vietnamiensis and G. diazotrophicus, b) to analyze the growth of Z. mays with B. vietnamiensis and G. diazotrophicus and the NH4NO3 at 50%.
This research was carried out in the Environmental Microbiology Laboratory of the Biological Chemical Research Institute of the Michoacán University of San Nicolás de Hidalgo, the average microclimatic conditions were: temperature of 23.2°C, luminosity of 450 μmol・m-2s-1, relative humidity of 67%.
Origin and preparation of the soil
Table 1 shows the main soil properties of the experiment: one of the sodium lateritic type; Clay texture, poor in the concentration of organic matter with 4.57% and total N (nitrogen), with a slightly acidic pH of 6.64. This soil was collected from an agricultural plot called “La cajita” of the Tenencia Zapata in the municipality of Morelia, Mich., on km 5 of the Morelia-Pátzcuaro highway, México, solarized at 75oC/48 h to minimize pest problems and diseases.24 Then a 1.0 Kg of soil was placed in the upper container of the Leonard jar, while in the lower part reservoir the NF or water, both parts were connected by a 25 cm long cotton strip for capillarity movement of the liquid to the ground (Figure 1).
Parameters |
Values |
Total, Nitrogen |
0.62 |
Total, phosphorus |
0.30 |
pH (1:20) |
6.64 |
Organic matter (%) |
4.57 |
Cation exchange capacity (C mol (+) Kg-1) |
46.1 |
Texture (%) |
20.56 (Cy)-37.8 (Si)-0.76(Sa) |
Density (g/cm3) |
2.01 |
Apparent density(g/cm3) |
1.08 |
Porosity** (%) |
46.35 |
Moisture saturation percentage (%) |
46.95 |
Field capacity*** (%) |
30.08 |
Useful humity (%) |
13.25 |
Table 1 Physicochemical properties of the soil for the growth of Zea mays with Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus endophytes of Stenocereus queretaroensis
Sa, Sandy; Si, Silt; Cy, Clay; *For soil of volanic origen, **Calculate from density and Apparent density; *** according texture type, + Reported for sandy loam soils NOM-021-RECNAT-2000.
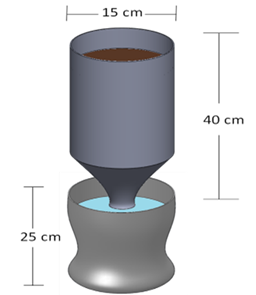
Figure 1 Diagram of the Leonard jar. (Selection of endophytic Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus from roots of Stenocereus queretaroensis).
Isolates of endophytic B. vietnamiensis and G. diazotrophicus were recovered from inside the root of S. queretaroensis; 5-cm sections were taken and washed with sterile water, disinfected with 70% alcohol/2.5 min, then with 10% NaClO/2.5 min; then they were macerated in a mortar with 10.0 mL of a saline solution (NaCl 0.85%) Roma™ detergent 0.01% (SSD), from which 1.0 mL was taken, and striked in Pseudomonas cepacia agar, azaleic acid with and without tryptamine (PCAAA) with the following composition (gL-1): tryptamine 0.2; azaleic acid 2.0; K2HPO4 4.0; KH2PO4 4.0; yeast extract 0.02; MgSO4 0.2; pH adjusted to 6.7; PCAAA was incubated at 28°C/72h; while to recover G. diazotrophicus, sucrose yeast extract agar (SYEA) was used with the following composition (gL-1): sucrose 100.0; K2HPO4 2.0; KH2PO4 2.0; NaCl 1.0; MgSO4 3.0; yeast extract 1.0; 10 mL bromothymol blue at 2.0% (p/v) (Cavalcante & Dobereiner, 1988) with 10 mL/L of the trace element solution with the following composition (gL-1): H3BO4 2.86; ZnSO4 0.22; MnCl2 1.81; K2MnO4 0.09; the pH was adjusted to 5.7; both PCAAA and SYEA were incubated at 30°C/72h;24 when round bright translucent colonies suspicious for B. vietnamiensis were detected, they were again growth in PCAAA to obtain an axenic culture, then short Gram-negative bacilli were registered by Gram stain; microscopic morphological characteristics common in B. vietnamiensis: while for G. diazotrophicus in the SYEA, bulging and bright round colonies with a yellow intracellular pigment were observed, while Gram-negative coccobacilli were detected under the microscope, which according to the literature belong to these genus.25–28
Growth of Zea mays with Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus endophytes and 50% of NF. Z. mays seeds were disinfected with NaClO at 3% (v/v)/5 min, washed six times with sterile water, then with alcohol at 70% (v/v)/5 min, washed six times with sterile water, later in plastic bags of 250 g for every 10 Z. mays seeds, were inoculated with 1.0 mL of B. vietnamiensis and G. diazotrophicus in a concentration that was adjusted to 1 x 106 CFU/mL, obtained by viable count on plate in PCAAA and SYEA; when both were inoculated, a 1:1 ratio was used; after the inoculation of the seeds with B. vietnamiensis and G. diazotrophicus were shaken for 1 h to ensure the entry of B. vietnamiensis and G. diazotrophicus to the seeds of Z. mays, then five seeds of cereal s were sown in each Leonard Jar. Z. mays with or without B. vietnamiensis and G. diazotrophicus; according to the experimental design in Table 2, randomized blocks with two controls, three treatments and six replicates: Z. mays without B. vietnamiensis and G. diazotrophicus irrigated only with water or absolute control (AC); Z. mays without B. vietnamiensis and G. diazotrophicus fed with the NH4NO3 at 100% or relative control (RC); Z. mays inoculated individual/in consortium with B. vietnamiensis and G. diazotrophicus with NF at 50%in a mineral solution for this cereal with the following chemical composition (gL-1): NH4NO3 12.0, KH2PO4 3.0, K2HPO4 3.5, MgSO4 1.5, CaCl2 0.1, FeSO4 0.5 mL/L, and a 1.0 mL/L trace element solution, pH adjusted to 7.0, in distilled water, while NH4NO3 or NF was reduced to 50% equivalent to 6 g L-1.24
*Treatments |
Burkholderia vietnamiensis |
Gluconacetobacter diazotrophicus |
water |
NH4NO3 |
Absolute control (AC) irrigated only water |
- |
- |
+ |
- |
Relative control fed(RC) NH4NO3 at 100% |
- |
- |
- |
100% |
Treatment 1 |
+ |
- |
- |
50% |
Treatment 2 |
- |
+ |
- |
50% |
Treatment 3 |
+ |
+ |
- |
50% |
Table 2 Experimental design to analyze the growth of Zea mays with Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus endophytes from root of Stenocereus queretaroensis with. NH4NO3 at 50%
+n=6; (+) = applied; (-) = no applied
The growth response variables of Z. mays with B. vietnamiensis and G. diazotrophicus were: the percentage of emergence (%), at the seedling and flowering level based on phenology: plant height (PH) and root length (RL); in the biomass: aerial fresh weight (AFW) and radical (RFW); with aerial dry weight (ADW) and radical (RDW). The experimental data obtained were subjected to ANOVA analysis of variance using Tukey’s comparative test of means (P ≤ 0.05), for which the statistical program Statgraphics Centurion29 was used.
Evidence of the presence of B. vietnamiensis and G. diazotrophicus in the stem and roots of Zea mays
The sensitivity profile of B. vietnamiensis and G. diazotrophicus was obtained before and after inoculating Z. mays. Using the Kirby and Bauer method for genera and species of Gram negative bacteria: Ampicillin, Cefotaxime, Ceftazidime, Cefuroxime, Pefloxacin, Tetracycline and Trimethoprim-Sulfamethoxazole, Cephalotin, Dicloxacin, Erythromycin, Gentamicin and Penicillin.30 Both B. vietnamiensis and G. diazotrophicus isolated from S. queretaroensis were grown on nutrient agar and on PCAAA and SYEA; As a reference, the sensidiscs were placed before inoculating the Z. mays seed, then they were incubated at 32°C/48 h, and the inhibition halos were measured according to the Kirby-Bauer method. Later, when Z. mays was inoculated with B. vietnamiensis and G. diazotrophicus and the cereal reached the level of seedling and flowering, the following were taken: 1.0 g of leaves, stem and/or roots each were disinfected and macerated as described in item b), then 1.0 mL of leaves, stem and root with B. vietnamiensis and G. diazotrophicus; it was grown with a sterile swab in nutrient agar, PCAAA and SYEA, then the antibiotic sensidisks were placed and incubated from 24 to 48 h/30oC. Inhibition halos were then measured according to the Kirby-Bauer method,31,32 and the sensitivity pattern of individual B. vietnamiensis and G. diazotrophicus was obtained to compare with the antibiotic sensitivity profile of each before inoculating the seed of Z. mays. Finally, to confirm that they belonged to B. vietnamiensis and G. diazotrophicus, specific biochemical tests were used to identify them as members of these bacterial groups.33–35
Table 3 shows the results of the emergence percentage of the Z. mays seeds treated with B. vietnamiensis and G. diazotrophicus and NH4NO3 at 50%, with 95% of emergence, which indicates that B vietnamiensis and G. diazotrophicus converted exudates from the seed, known as spermosphere effect, such as tryptophan into phytohormons to accelerate starch hydrolysis, to end embryo dormancy and to accelerate the rate of stem and root primordium emergence, as detected in Z. mays only treated with B. vietnamiensis or with G. diazotrophicus and FN at 50%.9,36 These percent emergence values were statistically different than 84% Z. mays without B. vietnamiensis or either G. diazotrophicus fed only of the NF at 100% used as relative control (RC).
*Tratamiento (T) |
Emergency percentage |
(AC) absolute control irrigated only water |
81b** |
(RC) relative control fed NH4NO3 at 100% |
84b |
(T1) B. vietnamiensis NH4NO3 at 50% |
92a |
(T2) G. diazotrophicus NH4NO3 at 50% |
89b |
(T3) B. vietnamiensis and G. diazotrophicus NH4NO3 at 50% |
95a |
Table 3 Percentage of emergence of Zea mays seeds treated with Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus and NH4NO3 at 50%
+n= 6, **Values with different letters indicate statistical difference according to ANOVA-Tukey P ≤ 0.05).
In Table 4, the growth of Z. mays with G. diazotrophicus and 50% NF is presented for seedling, where 17.5 cm of PH and 13.0 cm of RL were registered. Numerical values without statistical difference with those observed in Z. mays with B. vietnamiensis and G. diazotrophicus plus NF at 50%, according to various reports it is shown that these endophytic genera interact efficiently with wild hosts, compared with those that inhabit the phyllosphere and/or the rhizosphere of a wide variety of domestic plants.37 What supports, B. vietnamiensis and G. diazotrophicus after germination colonized and penetrated the roots of Z. mays through different mechanisms some known and other not yet discovered that occur during plant,38 inside the plant conduction system they converted some low molecular weight carbon compounds derived from the metabolism of photosynthesis into phytohormons,39 which accelerated and improved the functioning of the root system to maximize uptake of NF to 50%, without compromising the healthy growth of Z. mays.20 shown in an increase in the numerical values of the phenology that were statistically different from those registered when Z. mays grew without B. vietnamiensis and G. diazotrophicus and the NF at 100% or relative control (RC); which the following results 13.3 cm PH and 9.1 RL. The growth of Z. mays with B. vietnamiensis and G. diazotrophicus and the NF at 50% based on the biomass registered 1.4 g of AFW and 0.37 g of RFW, while 0.23 g of DAW and 0.09 g DRW, the above indirectly demonstrates that both B. vietnamiensis and G. diazotrophicus were inside the roots, in the conduction system, transformed compounds from photosynthesis into phytohormons, to induce the greatest uptake of NH4NO3 and to optimize the dose at 50%.12,23,40 These numerical values of the biomass were statistically different with the 0.9 g of AFW and the 0.24 g of RFW, with the 0.08 g of DAW and with the 0.02 g of DRW of Z. mays used as RC, which shows that based on the healthy growth of Z. mays, the positive effect of B. vietnamiensis and G. diazotrophicus in optimizing NH4NO3 at 50%.
*Treatment(T) |
Plant height (cm) |
Radical |
Fresh weight (g) |
Dry weight (g) |
||
Aerial |
Radical |
Aerial |
Radical |
|||
(AC) absolute control irrigated only water |
12.5c** |
8.9c |
0.7c |
0.19c |
0.04c |
0.02c |
(RC) relative control fed NH4NO3 at 100% |
13.3b |
9.1b |
0.9b |
0.24b |
0.08c |
0.02c |
(T1) B. vietnamiensis and NH4NO3at 50% |
14.9a |
9.6b |
1.0b |
0.29b |
0.14b |
0.07a |
(T2) G. diazotrophicus NH4NO3at 50% |
17.5a |
13.0a |
1.6a |
0.40a |
0.17b |
0.05b |
(T3) B. vietnamiensis and G. diazotrophicus NH4NO3at 50% |
15.7a |
12.8a |
1.4a |
0.37a |
0.23a |
0.09a |
Table 4 Growth of Zea mays with Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus in phenology and seedling biomass with NH4NO3 at 50%
+n=6; **Values with different letters indicate a statistical difference according to ANOVA-Tukey (P ≤ 0.05).
Table 5 shows the growth of Z. mays with B. vietnamiensis individually or in combination with G. diazotrophicus and 50% of NH4NO3 flowering; there, they registered 92.17 cm of PH and 28.12 cm of RL; while in Z. mays with G. diazotrophicus and 50%, of NH4NO3 90.93 cm AP and 26.95 cm RL were obtained. These numerical data indicate that B. vietnamiensis and G. diazotrophicus, after colonizing inside of the root system, transformed organic compounds from root physiology into phytohormons,22 inducing a dense and extensive root system, the roots of Z. mays had a higher exploration capacity to uptake NH4NO3 at 50% without compromising the healthy growth of Z. mays.19,21,41 The numerical values of the phenology of Z. mays treated with B. vietnamiensis and G. diazotrophicus were statistically different compared to the 74.41 cm RL and 21.05 cm RL of Z. mays without B. vietnamiensis and G. diazotrophicus and the NF at 100% or relative control (RC). Concerning the biomass of the growth of Z. mays with B. vietnamiensis and G. diazotrophicus and the NF at 50%, they registered 31.03 g of AFW and 8.91 g of DRW, as well as 5.97 g of DAW and 1.23 g DRW; like Z. mays with G. diazotrophicus and NF at 50%. This suggests that both B. vietnamiensis and G. diazotrophicus endophytes of S. queretaroensis, by invading the interior of the roots of Z. mays, converted metabolites related to root physiology into phytohormones that induced the maximum radical uptake for the optimization of NF at 50%.40–43 These numerical values of the biomass of Z. mays with B. vietnamiensis and G. diazotrophicus and the NF at 50% were statistically different with the 26.06 g of AFW and the 2.85 g of RFW, with the 2.91 g of ADW and with the 0.91 g of DRW from Z. mays used as RC, that fact suggests that B. vietnamiensis as G. diazotrophicus when they are inside the root transformed organic compounds producing during root physiology into phytohormones that activated and improved the capacity of the root system for maximum uptake and optimization of NH4NO3 at 50% , and even that the healthy growth of Z. mays.15,19,21
*Treatments (T) |
Plant height (cm) |
Radical |
Fresh weight (g) |
Dry weight (g) |
||
Aerial |
Radical |
Aerial |
Radical |
|||
(AC) absolute control irrigated only water |
71.13c** |
20.03b |
21.02b |
2.55b |
2.08c |
0.45c |
(RC) relative control fed NH4NO3 at 100% |
74.41b |
21.05b |
22.97b |
2.85b |
2.91b |
0.41c |
(T1) B. vietnamiensis NH4NO3 at 50% |
88.43a |
24.13a |
26.09b |
6.7a |
3.93b |
0.91b |
(T2) G. diazotrophicus NH4NO3 at 50% |
90.93a |
26.95a |
29.73a |
7.93a |
4.19a |
1.07a |
(T3) B. vietnamiensis and G. diazotrophicus NH4NO3 at 50% |
92.17a |
28.12a |
31.03a |
8.91a |
5.97a |
1.23a |
Table 5 Growth of Zea mays at flowering stage with Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus in phenology and biomass and NH4NO3 at 50%
+n=6; **Values with different letters had a statistical difference according to ANOVA-Tukey (P<0.05).
In Table 6, the sensitivity profile of B. vietnamiensis and G. diazotrophicus endophytes of S. queretaroensis is shown to demonstrate that healthy root, stem and leaf growth of Z. mays seedling and flowering level, according to the Kirby and Bauer method, registered sensitivity to: Cefotaxime, Tetracycline, Trimethoprim-Sulfamethoxazole, Cefalotin, Erythromycin, in contrast resistant to: Ampicillin, Ceftazidime, Cefuroxime, Pefloxacin, Gentamicin, and Penicillin. This sensitivity profile was compared to that obtained for B. vietnamiensis and G. diazotrophicus before inoculating Z. mays seed. This suggests that B. vietnamiensis and G. diazotrophicus invaded the xylem of Z. mays to transform organic compounds from root physiology into phytohormones,43,44 which allowed the maximum uptake of NF to 50% for healthy growth.45
Antibiotic |
Endophytic isolates from Stenocereus queretaroensis |
Recover from Zea mays |
||||||
B. vietnamiensis |
G. diazotrophicus |
B. vietnamiensis |
G. diazotrophicus |
|||||
Root |
stem |
leaf |
root |
stem |
leaf |
|||
Ampicillin |
- |
- |
- |
- |
- |
- |
- |
- |
Cefotaxima |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Ceftazidime |
- |
- |
- |
- |
- |
- |
- |
- |
Cefuroxime |
- |
- |
- |
- |
- |
- |
- |
- |
Pefloxacin |
- |
- |
- |
- |
- |
- |
- |
- |
Tetracycline |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Trimetoprim-Sulfamethoxazole |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Cefalotin |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Dicloxacin |
- |
- |
- |
- |
- |
- |
- |
- |
Erythromicin |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Gentamicin |
- |
- |
- |
- |
- |
- |
- |
- |
Penicillin |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Table 6 Antibiotic sensitivity profile of Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus recovered from roots, stem and leaf of Zea mays NH4NO3 at 50%
(+) = sensitivity; (-) = resistant.
Table 7 shows the biochemical profile of B. vietnamiensis and G. diazotrophicus endophytes of S. queretaroensis, based on a prototype strain and related literature; there, it is reported that the genus and species of B. vietnamiensis is a short bacillus Gram negative, a facultative aerobe that synthesizes catalase and oxidase; so it has an oxidative metabolism, it uses sugars: esculin, glucose, which do not ferment, but organic acids: sodium citrate; it is a solubilizer of PO4-3 by the generation of acid and alkaline phosphatases; They reduce NO3 to NO2. B. vietnamiensis fixes N2, grows in a mineral medium without any form of combined N;46,47 selectively uses tryptamine as a source of N; convert tryptophan into indole acetic acid and induces a dense and larger root system, which allowed Z. mays to uptake NH4NO3 at 50%.5,48,49 Gluconoacetobacter diazotrophicus of S. queretaroensis is an osmophilic endophytic genus and species similar to the prototype strain of G. diazotrophicus of S. officinarum (sugar cane). It has an oxidative metabolism of sugars: glucose, fructose and sucrose; uses citrate as the sole source of carbon and energy; by glucose fermentation it generates ethanol, lactate and acetic acid;50 fixes N2 in facultative endophyte association, grows in a culture medium without any source of organic or inorganic N, in anaerobiosis reduces NO3 to NO2; transform L-tryptophan into auxin, a phytohormone that generates a dense radical system that in Z. mays improved radical uptake of NH4NO3 and optimized the dose reduced at 50%.51–54
Biochemical test |
Burkholderia |
Gluconacetobacter diazotrophicus |
||
*Prototype |
**Endophytic isolated |
*Prototype |
**Endophytic isolated |
|
Gram stain |
- |
- |
- |
- |
Rod shape |
short |
short |
coccobacilli |
cocobcilli |
Esculin hydrolisis |
+ |
+ |
+ |
+ |
Glucose fermentation |
- |
- |
+ |
+ |
Vogues-Proskauer |
- |
- |
+ |
+ |
Xilose |
+ |
+ |
+ |
+ |
Sodium citrate |
+ |
+ |
+ |
+ |
Indole |
+ |
+ |
+ |
+ |
Urease |
+ |
+ |
- |
- |
arginine hydrolase |
+ |
+ |
+ |
+ |
Nitrate reduction |
+ |
+ |
+ |
+ |
Table 7 Biochemical profile of Burkholderia vietnamiensis and Gluconacetobacter diazotrophicus endophytes of Stenocereus queretaroensis in Zea mays and nitrogen fertilizer at 50%
+Prototype strain response. **Recovered from inside of S. queretaroensis; positive reaction (+); negative reaction (-).
We acknowledge support to the Project 2.7 (2022) from CIC-UMSNH also to Phytonutrimentos de México and BIONUTRA, S.A de CV Maravatío, Michoacán, México.
The authors state that there is no conflict of interest.
To Project 2.7 (2022) from CIC-UMSNH and to Phytonutrients de México and BIONUTRA, S.A: de CV Maravatio, Michoacán, México.

©2022 González-Campos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.