Journal of
eISSN: 2572-8466


Conceptual Paper Volume 6 Issue 3
1Kuban State Medical University, Russia
2FSBI “Federal Center for Cardiovascular Surgery” of the Ministry of Health of the Russian Federation, Russia
3Saint Petersburg Federal Research Institute of Physical Culture, Russia
Correspondence: Konstantin G Korotkov, St. Petersburg Research Institute of Physical Culture and Sport, NIIFK, Ligovski prospect 56E, St. Petersburg, 19104, Russia, Tel 7-9219368394
Received: April 13, 2019 | Published: May 2, 2019
Citation: Pokrovskii VM, Nechepurenko AA, Tarasov DG, et al. Sinoatrial node pacemaker cell pool dynamics upon synchronization with vagus nerve rhythm. J Appl Biotechnol Bioeng. 2019;6(3):114-116. DOI: 10.15406/jabb.2019.06.00182
Aim: To reveal the dynamics of sinoatrial node pacemaker cell pools upon synchronization with vagus nerve rhythm through the model of vagal-cardiac synchronization.
Materials and methods: Observations were carried out on 10 narcotized cats. The animals were tracheostomized and pump-ventilated, and the pericardium accessed via an open-chest transsternal incision. A device (KELSY scanner manufactured by Elsys, St. Petersburg, Russia) accompanied by a microscope and a video-camera, to visualize the luminescence of excitation processes in the sinoatrial node in a high-frequency electromagnetic field (1024 Hz) was placed in the sinoatrial area of a working heart. Luminescent focus in the sinoatrial node was registered as a peripherally cut end of the vagus nerve was stimulated with bursts of electrical impulses (5 impulses, 2 ms, 20 Hz) from an electrostimulator.
Results: Luminescence localized at the entrance of the cranial vena cava was visualized in a high-frequency electrical field in narcotized cats. The luminescent focal area was not homogenous and looked like a number of luminescent pools. Upon vagal-cardiac synchronization caused by the stimulation of a peripherally cut end of the vagus nerve with bursts of electrical impulses, the focus was wide and solid.
Conclusion: Here, pacemaker cell dynamics were studied in the feline heart. When vagal- cardiac synchronization was activated, synchronization of the heart with vagus nerve rhythm was accompanied by an increase in the early depolarization area in the sinoatrial area of the feline heart. The mechanisms underlying heart rate synchronization are not clearly defined. Rhythm is achieved through actions of the SA node and the vagus nerve. Our data confirm the vagal- cardiac synchronization model.
Keywords: sinoatrial node, pools of pacemaker cells, vagal-cardiac synchronization
Heart rhythm-generation research is significant to the cardiology field. According to traditional ideas, heart rhythm is initiated by pacemaker cells of the sinoatrial node (SA node). The extracardiac nervous system has an adjusting influence observed in the increased and decreased heat rate, which can manifest in two ways. Endocellular mechanisms can cause changes in the speed of slow diastolic depolarization in the action potentials of SA node pacemaker cells, or intercellular interactions can synchronize pacemaker cell-pools that have different rhythms, in order to attain one common rhythm.1,2 Some theories suggest that the synchronization rhythms of pacemaker cell pools achieve a single system of heart rhythm generation by means of nexuses, electrical, and electrical tonic interactions, but these theories are often contradictory and do not fully explain the process.2–4 Simultaneous incoming nerve impulses from the brain via parasympathetic fibers of the vagus nerve, i.e. synchronization of the SA node pacemaker with the rhythm of the vagus nerve, is a suggested mechanism of pacemaker cell-pool synchronization.This hypothesis is possible if we proceed from the hierarchical concept of rhythmogenesis.
According to V.M. Pokrovskii’s concept,5–9 heart rhythms in an organism are generated by a hierarchical system of structures and mechanisms that include heart and brain interaction. Rhythm is generated by a holistic brain, terminating in the final link of rhythm generation – efferent structures of the vagus nerve in the medulla oblongata. Signals, like bursts of nerve impulses that travel along the vagus to the SA node, are thought to originate in the medulla oblongata. These signals interact with automatic structures of the node and initiate heart rhythm. Vagal-cardiac synchronization is a model of synchronization of the sinoatrial nerve with vagal rhythms: in response to a burst of electrical impulses stimulating a peripherally cut end of the vagus nerve, the heart makes one contraction in a strictly defined period of time. A change in the frequency of bursts in a definite frequency range causes a synchronous change in heart rate.10,11 In this study, the dynamics of pacemaker cell pools in the SA node were observed utilizing pacemaker cell luminescence in a high-frequency electromagnetic field.
This study was approved at a meeting of the ethical Committee of the Kuban State Medical University. The authors of the study confirm that they have taken all measures to minimize the pain and suffering of animals in accordance with the main provisions of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. In the experiment 10 outbred adult cats from a University nursery were used, of both sexes, weighing 2.8-3.2 kg. All the animals were on a normal diet until the surgery. Induction into anesthesia was performed by intraperitoneal administration of sodium thiopental solution at a dosage of 40 mg/kg. Further introduction of the anesthetic was not provided by the study protocol. Tracheostomy was performed for the animal and it was transferred to mechanical ventilation. The vagus nerve was prepared in the neck area; nerves were incised after ligatures were made. The animals were tracheostomized and pump ventilated, and the pericardium accessed via an open-chest transsternal incision. A device (KELSY scanner manufactured by Elsys, St. Petersburg, Russia) used to visualize the luminescence of the excitation process in the SA node in a high-frequency electromagnetic field (1024 Hz) was placed into the sinoatrial area of a working heart. The device, which was developed on the basis of K.G. Korotkov’s research,1 was accompanied by a microscope and a video-camera. A Neiro-MVP-4 device (manufactured by the “Neirosoft” company, Ivanovo, Russia) was employed to record electrocardiograms (ECG) with the first standards ECG lead.
Luminescent focus was registered in the sinoatrial area; a peripherally cut end of the vagus nerve was stimulated with bursts of electrical impulses (5 impulses, 2 ms, 20 Hz) from an electrostimulator. This stimulation instigated vagal-cardiac synchronization – a phenomenon in which the heart makes one contraction to respond to every burst of electrical impulse that reaches it. Changes in the burst frequency caused a synchronous change of heart rate within a certain frequency range. Heart rate was measured during experimentation, and the early depolarization area in the SA node was characterized as either a pool or solid area. The size of the early depolarization area was measured, and the area was localized. Brightness of luminescence focus was calculated, and analysis included the range, maximum and minimum limits of wavelength, and the median values of wavelength. The experiment did not provide for the reuse of animals. At the end of the experiment the animals were not removed from the surgical stage of anesthesia. Animal’s skin was sutured and lung ventilation was turned off. The authors confirm that there were no signs of animals suffering.
Statistical analysis was performed employing STATISTIKA 6.0 for Windows software (Stat Soft, Inc). First, normal distribution was determined, and then parametric methods were employed. Arithmetical mean (M) and mean square deviation (SD) were calculated. Student’s t-test with p<0.05 was applied to determine whether coupled sets of mean values were significantly different.
Narcotized, pump ventilated cats with their chest and pericardium opened had an initial heart rate of 172.3±1.2bpm. Luminescence localized to the entrance of the cranial vena cava was visualized in the high-frequency electrical field, with the microscopic view showing that localization was not homogenous and looked like a number of luminescent pools (Figure 1). Vagal-cardiac synchronization is a model in which the heart adopts the rhythm of the vagus nerve. This model describes the phenomenon of the heart responding to every burst of electrical impulse that was incurred on the peripherally cut end of the vagus nerve with one contraction. A change in the frequency of bursts in a certain frequency range resulted in a synchronous change in heart rhythm (Figure 2).
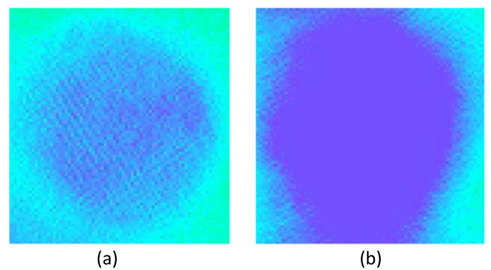
Figure 1 The luminescent focus (microscopically enhanced (40x15) and 7x software zoom) in the sinoatrial node of a feline heart in the initial state (a) and upon vagal- cardiac synchronization (b).
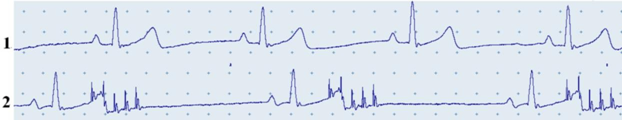
Figure 2 Electrocardiogram of a cat in the initial state (1) and upon vagal-cardiac synchronization (2). Record 2 also shows mark points for bursts of electrical impulses.
When vagal-cardiac synchronization was in action, the luminescent focus increased in area (Table 1) and became solid (Figure 1), with heart rate decreasing to 107.5±1.4bpm. The luminescent brightness of the initial depolarization area in the SA node increased 15.4% when vagal-cardiac synchronization was activated, which serves as indirect evidence of the increased number of cells involved in the excitation process (Figure 3). Emission of the initial depolarization area lay in the visible and ultraviolet spectral range. The wavelength range of the luminescence of the initial depolarization in the SA node increased 7.3% when vagal-cardiac stimulation was activated; the median value shifted towards longer luminescence waves. The increase of the wavelength range is apparently predetermined by a higher number of excited pacemakers pools not only in the center, but also on the periphery of the SA node (Figure 4).
Parameters |
Statistical indexes |
Initial |
Synchronization |
Luminescence focal area size, sm2 |
M±SDP |
0.040±0.009 |
0.172±0.016 |
Р<0.001 |
|||
Brightness histogram of the luminescence focal area, bit |
M±SDP |
130.0±4.4 |
150.0±3.2 |
Р<0.001 |
|||
Minimum limit of wavelength, nm |
M±SDP |
410±8.8 |
400.0±3.8 |
Р>0.05 |
|||
Minimum limit of wavelength, nm |
M±SDP |
232.7±10.0 |
250.0±3.5 |
Р<0.001 |
|||
Range, nm |
M±SDP |
232.7±10.0 |
250.0±3.5 |
Р<0.001 |
|||
Median value of wavelength, nm |
M±SDP |
550.4±8.5 |
500.0±3.8 |
Р<0.001 |
Table 1 Parameters of the luminescence focal area corresponding to the early depolarization area in the SA node of the feline heart at the initial state and at vagal-cardiac synchronization
This study revealed data on the synchronizing influence of the vagus nerve on the dynamics of pacemaker cell pools in the SA node. The data obtained in the experiment based on the central rhythmogenesis model (in which an electro stimulator acts as a generator nerve impulse bursts that are normally produced by brain) confirm the influence of the vagus nerve in the feline model. This study indicates that when vagal-cardiac synchronization is activated, the early depolarization area increases and becomes solid due to the united activity of pacemaker cell-pools. These facts give ground to the notion that in an intact organism, the generation of heart rhythm is a result of the interaction of discrete signals coming from the brain via the vagus nerve, with rhythm-generating structures of the SA node. Hence, on the output, the heart reproduces the rhythm of signals formed in the brain.10–13 Thus, when different pools of pacemaker cells simultaneously receive and adopt a signal via nerve, early depolarization areas become larger.
None.
The author declares there are no conflicts of interest.

©2019 Pokrovskii, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.