Journal of
eISSN: 2572-8466


Research Article Volume 6 Issue 6
1Bioenergetics and Environmental Sciences Division, India
2Department of Science &Technology, India3Acharya Institute of Technology, India
33 Acharya Institute of Technology, India
Correspondence: Manpal Sridhar, Bioenergetics and Environmental Sciences Division, ICAR-National Institute of Animal Nutrition and Physiology, Adugodi, Bengaluru 560 030, India
Received: November 07, 2019 | Published: December 2, 2019
Citation: Ravichandran A, Rao RG, Gopinath MS, et al. Purification and characterization of versatile peroxidase from Lentinus squarrosulus and its application in biodegradation of lignocellulosics. J Appl Biotechnol Bioeng. 2019;6(6):280-286. DOI: 10.15406/jabb.2019.06.00205
Versatile Peroxidases are high redox potential peroxidases capable of degrading lignin of lignocellulosic crop residues. Hence Versatile Peroxidases are prominent biocatalysts in upgrading lignocellulosic biomass for biotechnological applications. In the interest of exploiting the potential of Versatile Peroxidase in improving the digestibility of crop residues through delignification, a novel Versatile Peroxidase was purified and characterized from the immobilized cultures of native isolate Lentinus squarrosulus. The enzyme was purified with a specific activity of 62 U/mg through ion exchange and gel filtration chromatographic procedures. The enzyme possessed high affinity towards RB5 and manganese with a Km value of 6.84 µM for RB5 and 0.15 mM for manganese. The optimum temperature for oxidation was identified to be 30°C and optimum pH for manganese and RB5 oxidation was 5 and 3 respectively. Reactivity of the enzyme towards diverse substrates was investigated besides studying the effect of metal ions and inhibitors on RB5 oxidation. The enhanced potential of this purified Versatile Peroxidase in biodegradation of crop residues was demonstrated through augmentation of digestibility of finger millet and paddy straws by 20%.The results demonstrated that Versatile Peroxidase from Lentinus squarrosulus is capable of enhancing the nutritive value of crop residues through delignification.
Keywords: biodegradation, versatile peroxidase, natural substrate, lignin
WRF, white-rot fungi; VP, versatile peroxidase; LiP, lignin peroxidases; MnP, manganese peroxidases, Lac, laccase; RB5, reactive black; PUF, poly urethane foam; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin
Lignocelluloses are one of the abundantly existing economic and renewable biomass resources worldwide. Lignin degradation is central for efficient exploitation of these energy rich lignocelluloses. Research efforts on these lines have largely focused on White-rot Basidiomycetes for their selective degradation of lignin.1,2 Lignin degradation by Basidiomycetes is a multi-enzymatic process lead by ligninolytic enzymes of phenol oxidases and peroxidases. The urge for these ligninolytic enzymes has grown in recent years as they play an important role in great deal of biotechnological applications by their significant oxidation potential.3 A typical White-rot fungi (WRF) produces combination of these enzymes towards degradation of their natural substrate, the lignin. Diverse fungal species exhibit varying abilities towards lignin degradation majorly due to distinct efficiency of the underlying ligninolytic enzymes.4 In general, ligninolytic potential and activity of these enzymes differ among White-rot species and on the environmental conditions.5 This has ushered broad attention in recent years on identification of novel fungal isolates as a source of potential lignin degrading enzymes, as lignin degradation is strongly influenced by varying kinetics and catalytic properties of these enzymes. Versatile Peroxidases (EC 1.11.1.16) are distinct ligninolytic heme peroxidases with combined catalytic abilities of Lignin Peroxidases (LiP, EC 1.11.1.14) and Manganese Peroxidases (MnP, EC 1.11.1.13). A noteworthy feature is the catalysis by this enzyme unconditional of the redox mediators that is a major advantage for economic biotechnological applications.6 Accordingly, this study was carried out to understand the kinetics and catalytic potential of a novel Versatile Peroxidase(VP)from Lentinus squarrosulus. Lentinus squarrosulus is a tropical white-rot that predominantly produces Laccase (Lac) and VP in manganese deficient medium.7 Consequently, VP was purified from culture medium optimized for production of the enzyme and studied for its kinetics, substrate preferences and catalytic properties. Thermal and pH stability are major determinants of a competent industrial biocatalyst and in view of this, stability properties of VP from Lentinus squarrosulus was also evaluated in this study. The purified enzyme was then exploited for biodegradation of crop residues to appreciate the enzyme’s potential on fiber degradation and in vitro dry matter digestibility.
Fungal strain and culture conditions
The White-rot Basidiomycetes used in this study was Polyporales Lentinus squarrosulus, a native strain of Western Ghats, India. The fungus was collected from decayed wood logs and identified as a producer of VP through our initial analysis.7 Pure culture of the fungus was maintained at 4°C on potato dextrose agar and seed culture was prepared in potato dextrose broth from freshly grown culture when required. For production of VP, Lentinus squarrosulus immobilized on poly urethane foam (PUF) cubes was used. Fungus from seed culture was homogenized and added to potato dextrose broth containing 20% PUF cubes and incubated at 35ºC under continuous agitation of 80 rpm. After sufficient establishment of growth on PUF cubes, the cells were washed with saline and the nutrient medium for growth was replaced with the medium optimized for production of VP. The optimized production medium comprised per liter: Glucose 25 g/l, Yeast extract 0.25 g/l, veratryl alcohol 0.6 g/l, DMP 0.6 g/l and trace elements 1 ml. Culture supernatant was harvested on the day of maximum enzyme activity.
Enzyme purification
Culture fluid was harvested after 5 days and filtered to remove any solid particles. The culture supernatant was then subjected to 65% ammonium sulphate fractionation at 4°C and subsequently protein precipitate was dissolved in 20mM sodium acetate buffer pH 4.5.The protein solution was then dialyzed against 10 mM sodium acetate buffer pH 4.5 for removal of salts and applied to Q Sepharose fast flowanion exchange column equilibrated with sodium acetate buffer pH 4.5. Retained proteins were eluted by applying linear gradient of 0.2-1M NaCl at a flow rate of 0.1 ml/min. Fractions possessing VP activity was pooled and applied to Sephadex G-75 gel filtration column equilibrated with acetate buffer pH 4.5. Proteins were eluted using sodium acetate buffer (pH 4.5) at a flow rate of 1 ml/min. Fractions were assayed for protein by monitoring the absorbance at 280 nm and enzyme activity through RB5, manganese oxidation assays. Pooled fractions with VP activity was concentrated on Amicon PM 10 membrane and stored at -20°C.
Enzyme activities
VPactivity was determined by monitoring manganese oxidation and RB5 decolorization. Manganese oxidation was estimated by following the linear increase in absorbance of the Mn3+ malonate complex at 270 nm. Reaction mixture contained 0.5mM manganese in sodium malonate buffer pH 4.5. RB5 decolorization was observed with 10µM RB5 in sodium tartrate buffer pH 3. Reactions were initiated with 0.1mM H2O2.VP activity towards non-phenolics was monitored using 2mM of veratryl alcohol in pH 3 sodium tartrate buffer. Activity of the enzyme towards phenolics was quantified using 0.4 mM 2, 6 dimethoxy phenol, 0.1 mM ABTS and 35 µM vanillyliden acetonein pH 3 sodium tartrate buffer at the absorption maxima of the corresponding substrate. One unit is defined as the amount of enzyme that transforms 1 µmol of substrate per min.
Enzyme kinetics
Steady state kinetics of purified VP with Reactive Black 5 and manganese was studied. Reactive black 5 concentrations were varied from 2-20 µM whereas manganese concentration was varied from 0.05-2.5mM. Kinetic constant for manganese peroxidase activity was calculated from manganese oxidation assay at 270 nm and for manganese independent activity on RB5 through the dye decolorization examined spectrophometrically at 598 nm. The mean value of reaction rates was plotted on to Lineweaver-Burk plot and steady state kinetic constants obtained. Effect of 0.25 mM manganese on the kinetics of RB5 oxidation by the enzyme was also studied.
Enzyme characterization
The reactivity of purified VP towards diverse substrates like Reactive Black 5, manganese, 2, 6dimethoxy phenol, vanillylidenacetone, MBTH (3-methyl-2-benzothi-azolinone hydrozone)-DMAB (3-(dimethylamino)benzoic acid)and veratryl alcohol was examined at specific assay conditions. Oxidation of the substrates was monitored at pH 3 except for manganese at pH 4.5.Oxidation of calorimetric dye Leucoberbelin blue at 0.04% was studied at 620 nm.8 The effect of metal ions Cu, Zn, Fe, Mg, K and Ca at 1 mM and 0.25 mM concentrations on manganese independent peroxidase activity was evaluated and expressed as relative activity by comparison with activity in presence of RB5 only. Influence of pH on RB5 oxidation was determined using sodium tartrate buffer (pH 2.5 -5), phosphate buffer (pH 5.5-8.5), carbonate buffer (9-9.5) and on manganese oxidation using sodium malonate buffer (pH 3.5-6). The effect of reaction temperature on RB5 oxidation was monitored in the temperature range of 30°C to 50°C by incubating the reaction mixture at specific temperature for constant time interval. Thermal stability of purified VP was evaluated by exposing the enzyme to temperatures ranging from 60°C to 85°C for time span of 20 to 120 mins. Purified enzyme was incubated at preset temperature in water bath and aliquots of enzyme were withdrawn at specific time intervals, chilled on ice and activity determined through manganese oxidation assay. Residual activity was measured after the specified time and expressed as % relative activity with unincubated enzyme as reference. N-Bromo succinimide (NBS) and DMSO-HCl were tested as inhibitors of VP activity. Purified VP was incubated with 5 mM NBS in 100 mM sodium acetate buffer pH 4.0 for 30 mins at room temperature.9 The sample was then dialyzed against 10 mM sodium acetate buffer pH 4.0 for three hours and utilized for assay of residual enzyme activity. To determine the effect of DMSO-HCl, purified enzyme was incubated with 10µl DMSO in 200 µl acetic acid and 100µl 12N HCl for 30 mins at room temperature.10 Protein was then dialyzed against 10 mM sodium acetate pH 4.5 and utilized for enzyme assay. Residual enzyme activity was assayed through RB5, manganese and ABTS oxidation. Molecular weight of the purified VP was estimated by sodium dodecyl sulfate- polyacrylamide gel electrophoresis with 12.5% resolving gel using Bio-Radprecision plus protein marker.11
Lignocellulose biodegradation
The lignocellulosicsubstrates finger millet straw and paddy straw(1mm x 1mm) were incubated with the purified enzyme at 25mU/g of straw for 24 hours, dried at a constant temperature of 60°C and then analyzed for proximate principles. Neutral detergent fiber (NDF), Acid detergent fiber (ADF) and Acid detergent lignin (ADL) were analyzed through methodology of Van Soestet al.12 In vitro dry matter digestibility was evaluated using two stage digestion method.13
Dye decolorization
Sulfonephthaleindyes bromophenol blue and bromocresol green, triaryl methane dye cresol red and phenothiazinium dye Azure B were observed for decolorization by purified VP. Dye at a final concentration of 1 mM was incubated with 20mU of enzyme and reaction mixture was maintained at pH 3. Reaction was initiated with 0.1 mM H2O2. Rate of decolorization was monitored at specific time intervals at the absorbance maximum of the corresponding dye.
Lentinus squarrosulus established itself profoundlyon inert PUF matrix with Laccase and VP being the major lignin modifying enzymes detected in the production medium. In the production phase of immobilized culture, VP activity attained a maximum value of about 280 U/L after five days.
Versatile peroxidase purification
VP was purified from immobilized culture on the 5th day of production phase. After precipitating the proteins through salt precipitation, proteins were fractionated through Q Sepharose fast flow anion exchange chromatography using salt gradient. VP eluted as major peak at a gradient of 0.6M NaCl. Pooled fractions possessing activity were applied to Sephadex G-75 gel filtration column and eluted using sodium acetate buffer pH 4.5. Fractions holding VP activity were collected at an elution volume of 70 ml (Figure 1). Protein was purified with a yield of 19% and purification fold of 26% as presented in Table 1.
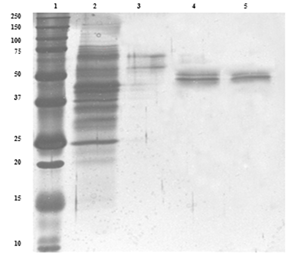
Figure 1 Denaturing electrophoretic analysis of samples stained with silver nitrate. 1) Bio-rad precision plus protein marker 2) Peroxidase from Bjerkanderaadusta (Sigma-aldrich) 3) Laccase from Trametes versicolor 4,5) Versatile Peroxidase from Lentinus squarrosulus.
Purification Step |
Protein (mg) |
Total Activity* (U) |
Specific Activity (U/mg) |
Purification fold |
Yield % |
Crude Filtrate |
83.2 |
195.2 |
2.35 |
1 |
100 |
Salt Precipitation |
44.2 |
167.7 |
3.79 |
1.62 |
85.91 |
Q Sepharose |
2.1 |
55.22 |
26.3 |
11.21 |
28.29 |
Sephadex G-75 |
0.6 |
37.26 |
62.1 |
26.47 |
19.09 |
Table 1 Purification summary of Lentinuss quarrosulus Versatile Peroxidase
*Activity values are 1based on manganese oxidation assay.
Enzyme kinetics
Kinetic constants of purified VP were studied with RB5 and manganese as substrates. The reaction rates at varying substrate concentration were studied and observed to follow typical Michaelis-Menton kinetics (Figure 2). At constant enzyme concentration, the rate of oxidation of RB5 increased proportionately up to 7µM, after which addition of more substrate had negligible effect on enzyme activity. The enzyme expressed high affinity towards RB5 as indicated by the affinity constant (Km) of 6.84 µM. Maximal reaction velocity with RB5 was 0.027 U/ml. Purified VP oxidized manganese efficiently with maximum reaction rate of0.28 U/ml and affinity constant (Km) of 0.15 mM. However, in the presence of 0.25 mM manganese, the reaction velocity of RB5 oxidation decreased in comparison to oxidation in the absence of manganese without any change in the enzyme’s affinity towards the aromatic substrate, RB5. This plausibly suggests that manganese is occupied at a different location other than the active site of oxidation of RB5. The kinetics of RB5 oxidation with manganeseimplieslatter as a non-competitive inhibitor of RB5 oxidation (Figure 3).
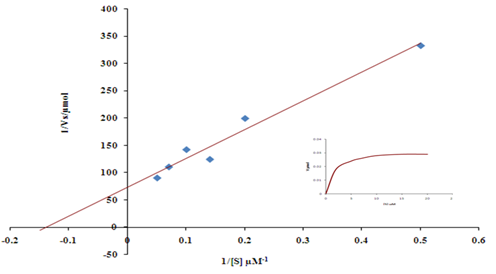
Figure 2 Lineweaver -Burk plot of RB5 oxidation by purified Versatile Peroxidase. Kinetics of RB5 oxidation denoted inset.
pH optimum for enzyme activity
Influence of pH on manganese and RB5 oxidation by VP were studied employing different buffers in the pH range 3.5 – 6 for the former reaction and 2.5 - 9.5 for the latter. The pH activity profile of manganese oxidation displayed an optimum at pH 5 and the activity was comparable in the slightly acidic pH range (Figure 4(A)). The RB5 oxidation activity decreased considerably as the pH scale moved towards neutral and negligible activity was detected with phosphate buffer (Figure 4(B)). Activity maximum was observed at acidic pH at an optimum value of 3. The optimal pH values observed for Lentinus squarrosulus VP is in concordance with VP from other species.14
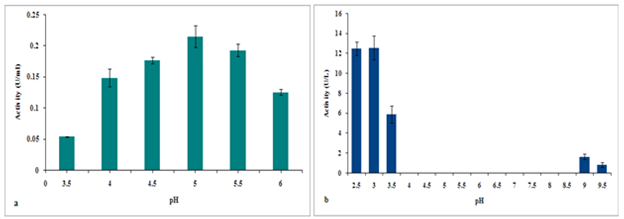
Figure 4 (a) pH profile of manganese oxidation by the purified enzyme (b) pH profile of RB5 oxidation by the purified enzyme.
Effect of temperature on enzyme activity
Optimum temperature for oxidation of RB5 by the purified VP was 30°C. The activity decreased by 40% on every 5°C increase in temperature till 40°C and on further increase of temperature till 50°C, there was no significant variation in enzyme activity. To assess the thermal stability, the purified enzyme was incubated at temperatures from 60°C to 85°C and residual activity was measured after specific time intervals. At an incubation time of 20 mins, nearly 40% of enzyme activity was retained at temperatures from 60°C to 70°C. Enzyme inactivation was prominent as the time progressed beyond 20 mins with only 20% activity after an hour at 60°C and 14% of initial activity at two hours. The same trend was observed till 75°C and at temperatures beyond 75°C, enzyme activity decreased logarithmically with less than 10% activity after two hours. At a temperature of 85°C, enzyme completely lost its activity after 80 mins and nearly 10% activity was retained at 60 minutes (Figure 5).
Effect of metal ions on enzyme activity
Effect of few cations at 0.25 and 1 mM concentration on RB5 oxidation activity of purified VP were studied at standard assay conditions. Ca2+ at 0.25 mM concentration reduced the activity by about 71% whereas at 1 mM concentration activity completely diminished. Cu2+ and Fe2+ were strongly enhancing the enzyme activity at 0.25 mM concentration, the activity nearly doubled with Cu2+at this concentration. However, the activity with these cations at 1 mM concentration was less significant than at 0.25 mM. With Zn2+and Mg2+, there was no change in activity at 0.25 mM and activity reduced as the Mg2+concentration was increased to 1 mM. Activity in presence of 1 mM Zn2+ slightly induced the activity. With K+, activity decreased to some extent irrespective of the concentration used (Figure 6).
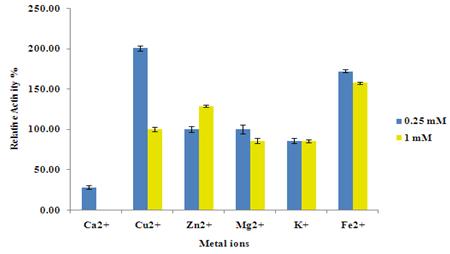
Figure 6 Effect of metal ions at different concentrations on RB5 activity by purified Versatile Peroxidase.
Substrate specificity and effect of inhibitors
Reactivity of purified VP with seven substrates was studied and activity towards these substrates is presented in Table 2. Activity towards ABTS at the concentration studied was the highest followed by manganese and 2, 6 dimethoxyphenol. Leucoberbelin blue (LBB) was used to follow manganese oxidation as this berbelin blue leuco base is reportedly oxidized only by manganese at higher oxidation states. However in our study, this dye was directly oxidized by the purified enzyme without addition of manganese to the reaction mixture yet, purified Versatile Peroxidase activated with H2O2 didn’t oxidize the dye instantly. With addition of manganese to Versatile Peroxidase subsequent to H2O2 at pH 4.5 bright blue product was visualized. Inclusion of EDTA prior to LBB in the former reaction mixture inhibited the oxidation reaction by chelating free manganese. To determine the reason for direct oxidation of LBB by Versatile Peroxidase, 2 mM of ferrous sulphate and 3 mM of ferric chloride hexahydrate were allowed to react with the dye in separate reactions. Oxidation of LBB as indicated by bright blue color was observed only with ferric chloride. The role of N-bromosuccinimide and DMSO-HCl as potential inhibitors of VP activity was studied at an inhibitor concentration of 5 mM and 80 mM respectively. VP reacted with NBS retained only 20% of the RB5 oxidation activity whereas with manganese oxidation there was no significant difference. With respect to DMSO-HCl, RB5 oxidation activity drastically decreased to 25% in addition to reduction of manganese oxidation activity by 17%. With both the inhibitors, the purified VP considerably lost the oxidation potential towards ABTS.
Substrates |
Activity (U/mg) |
Veratryl alcohol |
3.58 |
2,6 dimethoxy phenol |
31.93 |
Reactive Black 5 |
4.44 |
Mn2+ |
46.3 |
Vanillylidenacetone |
9.18 |
2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
73.89 |
MBTH-DMAB |
3.06 |
Table 2 Activity of purified Versatile Peroxidase with different substrates
Lignocellulose biodegradation
Biodegradation of lignocellulosic substrates fingermillet straw and paddy straw by the purified enzyme was evident by reduction in NDF by 6-8% and ADF by 4-8%in comparison to untreated straw. Acid detergent lignin was 17% less than the control for finger millet straw and 11% than control for paddy straw. To see the significance of lignin reduction on the straws, in vitro dry matter digestibility was estimated. Digestibility of finger millet straw rose by 18% with 17% decrease in lignin and paddy straw by 20% with 11% decline in lignin.
Dye decolorization
Cresol red was more susceptible to degradation by the enzyme followed by bromophenol blue. Decolorization was slightly slow during the initial 4 hours for Azure B after which there was significant decolorization observed till 12 hours. With bromocresol green, though there was decrease in concentration after 4 hours, observation at initial hours was contrary with increase in absorbance (Figure 7).
Extracellular ligninolytic enzymes Laccase, Lignin Peroxidase, Manganese Peroxidase and Versatile Peroxidase secreted by the White-rot fungi makes them unique in selective lignin degradation. The catalytic properties of these enzymes vary with species and its ecological niche. Accordingly, this study reports the characteristics of a novel VP purified from wild isolate Lentinus squarrosulus of Western Ghats, India. The isolate was identified as a producer of Laccase and VP in manganese deficient medium through our previous studies.7 Subsequently, we optimized the culture medium for enhancing the activity of VP and our results demonstrated that this enzyme is stimulated in medium with high carbon: nitrogen ratio. Ligninolytic enzyme production is a complex process and hence proper synchronization of the parameters is necessary to achieve maximum enzyme activity.15,16 To this effect, we immobilized the fungal cells on polyurethane foam (PUF) cubesas immobilization allows for easy manipulation and monitoring of the process parameters.17 With immobilization, slight agitation was sufficient for oxygen dispersion as the surface area available for oxygen contact is increased. In addition, repeated batches of enzyme can be harvested without drop in activity. Medium optimized for production of VP was added to immobilized fungal cells and the activity profile was monitored. Maximum activity was detected on 5th day of production with VP being the major enzyme. Enzyme was purified from the culture supernatant on the day of maximum activity with 26 fold purification through anion and gel filtration chromatographic procedures. The purified protein exhibited a molecular mass of 49 KDa, slightly higher than VP from other species reported.18,19 However, marginally higher molecular weight enzymes were perceived in cultures with immobilized fungus.20 VP from Lentinus squarrosulus was able to oxidize RB5 in manganese independent reaction with high affinity towards the bulky substrate as demonstrated by the Km value 6.84µM. Km value for manganese although slightly higher than RB5, was within the range reported for Versatile Peroxidases from other species.19,21 The observation that addition of manganese decreased the velocity of oxidation of RB5 besides maintaining the affinity towards the latter indicates that the cation doesn’t compete with the aromatic substrate for active site and probably occupies a different binding site. This fact is line with VP from Bjerkanderaadusta and Pleurotuseryngii specifying noncompetitive inhibition of RB5 by manganese.22 The optimum pH for RB5 and manganese oxidation was in the acidic range as for other ligninolytic peroxidases.14 The pH profile of RB5 oxidation revealed stringent conditions with activity decreasing rapidly beyond the optimal pH;however, manganese oxidation varied only moderately in the pH range 4-6. With respect to thermal stability, temperature further than 40°C is not conducive for the enzyme. Lentinus squarrosulus is thermo tolerant with optimum temperature for growth around 35°C, surprisingly VP produced by this fungus didn’t exhibit significant thermal stability. This could be attributed to the structural calcium ions of the enzyme that are lost at increasing temperatures.23 This enzyme was able to oxidize a wide range of substrates including 2, 6 dimethoxyphenol, ABTS, reactive black 5, vanillylidenacetone, MBTH-DMAB, Leucoberbelin bluein manganese independent reactions.Leucoberbelin blue and MBTH-DMAB coupled reactions are said to require oxidized forms of manganese.24 However, in our study, we observed direct oxidation of the compounds without dependence on manganese. Leucoberbelin blue was oxidized by ferric iron in our study whereas ferrous iron failed to oxidize the dye. The same behavior was observed with purified Versatile Peroxidase that has ferric heme center(Fe3+) which oxidized LBB irrespective of activation by H2O2. Activated Versatile Peroxidase has oxo ferryl (Fe4+) heme hence failed to oxidize the dye.6 Addition of manganese to this activated Versatile Peroxidase produced blue color signifying catalytic conversion of Mn2+ to Mn3+ by the enzyme. Manganese oxidation by peroxidases can be exemplified through this method. To correlate to the fact that Versatile Peroxidase employs exposed surface tryptophan residue in catalysis of recalcitrant substrates, we incubated purified enzyme with N-bromosuccinimide, a reagent that oxidizes tryptophan. The modified enzyme significantly lost the ability to oxidize RB5 yet manganese catalysis is unaffected standing to the fact that manganese is reacted at site different from RB5. Similarly, DMSO in aqueous HCl is also reported to modify tryptophan.10 Accordingly, in our study, purified enzyme reacted with DMSO-HCl was unable to catalyze RB5 oxidation. In contrast to NBS, there was slightreduction in manganese oxidation with DMSO-HCl for some unknown reasons. To ascertain the potential of purified VP in enhancing the digestibility of crop residues, the enzyme was applied to commonly available crop residues paddy and finger millet straw. 25 mU of enzyme was capable of degrading 17% lignin in finger millet straw and 11% lignin in paddy straw. The pragmatic effect of lignin degradation was substantiated through increase in digestibility of crop residues by 18-20%. Though application of VP in biotechnological applications like xenobiotic degradation, paper pulp bleaching etc. are described, role of VP in upgrading the digestibility of crop residues for better animal nutrition is not reported.6 Digestibility with this purified Versatile Peroxidase can be further enhanced through treatment with increased titers of the enzyme and refinement of conditions of the reaction. Augmentation of digestibility by VP is thus delineatedthat could be promising in improving the nutritive value of abundantly available crop residues towards upgrading animal nutrition.
This study discussed the purification of VP from Lentinus squarrosulus produced by immobilized fungal cells. Kinetics of the enzyme towards its two different substrates, aromatic molecule RB5 and manganese was determined followed by establishment of its reaction characteristics. The purified enzyme was subsequently studied for its potential in crop residue degradation and dye decolorization. The results implied the promising role of VP in enhancing the digestibility of crop residues.
The financial assistance by Department of Science and Technology (DST), Ministry of Science and Technology, Govt. of India, under the WOSA scheme (SR/WOS-A/LS-32/2016) is gratefully acknowledged by the first author. The authors thank the Director, ICAR - National Institute of Animal Nutrition and Physiology, Bangalore (Karnataka) India, for providing the necessary facilities to carry out the research work.
All authors declare no conflict of interest.
None.

©2019 Ravichandran, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.