Journal of
eISSN: 2572-8466


Mini Review Volume 1 Issue 3
1Department of Chemical and Biomolecular Engineering, University of Tennessee, USA
2Biosciences Division, Oak Ridge National Laboratory, USA
3BioEnergy Science Center, USA
4UT-ORNL Joint Institute for Biological Science, USA
5Department of Forestry, University of Tennessee Institute of Agriculture, USA
Correspondence:
Received: November 13, 2016 | Published: December 30, 2016
Citation: Meng X, Yoo CG, Li M, et al. Physicochemical structural changes of cellulosic substrates during enzymatic saccharification. J Appl Biotechnol Bioeng. 2016;1(3):87-94. DOI: 10.15406/jabb.2016.01.00015
Enzymatic hydrolysis represents one of the major steps and barriers in the commercialization process of converting cellulosic substrates into biofuels and other value added products. It is usually achieved by a synergistic action of enzyme mixture typically consisting of multiple enzymes such as glucanase, cellobiohydrolase and β-glucosidase with different mode of actions. Due to the innate biomass recalcitrance, enzymatic hydrolysis normally starts with an initial fast rate of hydrolysis followed by a rapid decrease of rate toward the end of hydrolysis. With majority of literature studies focusing on the effect of key substrate characteristics on the initial rate or final yield of enzymatic hydrolysis, information about physicochemical structural changes of cellulosic substrates during enzymatic hydrolysis is still quite limited. Consequently, what slows down the reaction rate toward the end of hydrolysis is not well understood. This review highlights recent advances in understanding the structural changes of cellulosic substrates during the hydrolysis process, to better understand the fundamental mechanisms of enzymatic hydrolysis.
Keywords: glucanase, cellobiohydrolase, β-glucosidase, lingocellulosics, fucose
DP, degree of polymerization; CrI, crystallinity index; NMR, nuclear magnetic resonance; IR, infrared; XRD, X-ray diffraction; GPC, gel permeation chromatography; MW, molecular weight; DA, dilute acid
Converting renewable lignocellulosic substrates such as biomass to biofuel has drawn tremendous amount of attention due to the growing concerns about environmental stewardship and diminishing availability of fossil fuels.1 Currently, the bioconversion process involves several steps: size reduction, pretreatment, hydrolysis, fermentation and distillation/purification. Pretreatment is known to increase cellulose accessibility by changing the chemical composition and physical structures of biomass plant cell wall and numerous physical or chemical pretreatments have been widely studied and reviewed in recent literatures.2,3,4 Enzymatic hydrolysis represents a process in which polysaccharides are effectively broken down to its corresponding sugar monomers by using a multi-component enzyme system and this is a very slow process in most cases due to the complex characteristics of lingocellulosics to protect its polysaccharides from de-polymerization. Years of research efforts have tried to correlate the changes of substrate factors caused by pretreatment to the changes in biomass recalcitrance reflected by the sugar yield after hydrolysis, however the exact molecular mechanisms of biomass recalcitrance are still not well-understood and much of the literature has reported inconsistent results.5 There is no doubt that the initial hydrolysis rate or the final hydrolysis yield is influenced by physicochemical structural features of lignocellulosic substrates. It has been reported that low cellulose degree of polymerization (DP), crystallinity (CrI), lignin content and high cellulose accessible surface area favor enzymatic hydrolysis.6,7,8,9 On the other hand, a rapid decrease in the conversion rate was repeated observed as the hydrolysis time extended. The exact mechanism leading to this rate decrease is not fully clear due to the complexity of the lignocellulosic substrate and enzyme system. Some studies have reported that the increased recalcitrance or the decreased reactivity of the substrate during hydrolysis was the major reason causing the gradual decrease of hydrolysis rate, while others suggested that negative mode of enzyme action such as inactivation, diffusion constraints and jamming were the potential factors to be responsible.10 Thus, this review focuses on the recent advances in understanding of limitations occurring during enzymatic hydrolysis that might be responsible for the gradual slowing down of the reaction, which will be extremely helpful to strengthen the understanding of intrinsic hydrolysis reaction mechanisms.
Structure and composition of lignocellulosic biomass plant cell wall
Lignocellulosic biomass represents one of the few resources that can facilitate sustainable production of substantial amount of biofuels. It contains three major constituents: cellulose, hemicellulose and lignin. Elementary fibril primarily cellulose containing ~36 β-D-glucan chains coated with other polysaccharides to form micro fibrils (Figure 1). Each micro fibril might contain up to 40 cellulose chains and is about ~10-20 nm in diameter. These fibrils are then cross-linked by hemicellulose and pectin matrixes to form macro fibrils which have the function to mediate structural stability in the plant cell wall.11 The spaces between cellulose and pectin matrixes are filled with lignin which covalently links to hemicellulose, providing mechanical strength to the plant cell wall. Other components known as extractives including fats, phenolics, resins, minerals are also present in lignocellulosic biomass.
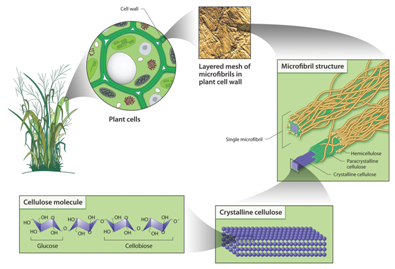
Cellulose contains highly ordered regions known as crystalline part as well as less ordered regions (amorphous). Cellulose chain has a strong tendency to form inter and intra-molecular hydrogen bonds among the sugar subunits within and between cellulose chains, promoting aggregation into a crystalline structure.12 The ultra structure of cellulose I, the most abundant form found in nature, is a mixture of two distinct crystal forms cellulose Iα (triclinic) and Iβ (monoclinic).13 The relative amounts of cellulose Iα and Iβ vary with the source of the cellulose, with Iα and Iβ being rich in the cell wall of primitive microorganisms and plants, respectively. In addition, it has also been suggested that cellulose contains a portion of para-crystalline which can be described as chain segments having less order but more mobility than crystalline segments.14 On the other hand, amorphous cellulose contains accessible fibril surfaces which are those in contact with water and inaccessible fibril surface portion representing those fibril-to-fibril contact surfaces as well as surfaces due to distortions in the fibril interior. Crystallinity index (CrI) is typically used to describe the relative amount of crystalline portion in cellulose and can be measured using several analytical techniques including X-ray diffraction (XRD), solid-state 13C nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy. The number of glucose units that make up one polymer molecule is defined as the degree of polymerization. Cellulose DP varies from ~250-500 in regenerated cellulose fibers to ~10000 in cotton fibers. It can be measured by various analytical techniques including viscometry and gel permeation chromatography (GPC).
Intimate contact between enzymes and cellulose is the prerequisite for enzymatic hydrolysis to occur. The crystalline structure of cellulose presents a great challenge to efficient hydrolysis, as it becomes difficult for enzyme to get access to the water insoluble and highly ordered nature of crystalline cellulose. The lignin and hemicellulose matrixes represents another barrier to efficient enzymatic hydrolysis because they can physically limit the access of enzymes to the activated surface of cellulose. Unlike cellulose, hemicellulose is a heterogeneous class of polymers representing a family of polysaccharides which may contain arabinose, xylose, mannose, glucose, uronic acid and fucose. The nano-space formed between cellulose and hemicellulose, approximately 5-10 nm in diameter, has been shown to represent the most fundamental barrier to enzymes in terms of biomass porosity.15 The significance of hemicellulose removal in enzymatic hydrolysis of biomass has been elevated in several recent studies.16,17,18 Lignin, an amorphous and 3D cross-linked polyphenolic polymer, can also physically limit the cellulose accessibility to enzymes. In addition, lignin also reduces the effectiveness of hydrolysis by binding cellulase unproductively thus decrease the availability of active enzymes.19
Enzymatic hydrolysis of cellulose
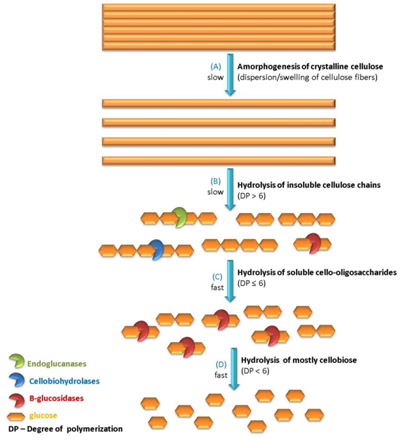
Cellulase represents the type of enzymes produced by fungi or bacteria that catalyzes the decomposition of cellulose-like polysaccharides. Three major types of enzymes are generally involved in hydrolyzing cellulose micro fibrils in plant cell wall: endo glucanase, exo glucanase and β-glucosidase. Endoglucanases, e.g., 1,4-β-D-glucan-4-glucanhydrolase could randomly attack the internal bonds at amorphous regions of cellulose while creating new chain ends that can be attacked by other types of enzymes. It is generally believed very active against soluble cellulose derivatives or acid-swollen amorphous cellulose.20 Exoglucanase, e.g., 1,4-β-D-glucan-cellobiohydrolases, generates glucose or cellobiose from the reducing or non-reducing end of cellulose polysaccharide chains. Unlike endoglucanase, exoglucanase is normally very active against crystalline cellulosic substrate such as Avicel or cello-oligosaccharides.18 Last but not least, β-glucosidase, e.g., β-glucoside-glucohydrolases, can hydrolyze cellobiose to glucose from the non-reducing ends and it is inactive against amorphous or crystalline cellulose. Figure 2 illustrates a proposed mechanism for cellulose de-polymerization by complex cellulase system. It is worth mentioning that fragmentation of cellulose aggregations into short fibers has been observed and reported during the beginning of cellulose hydrolysis prior to releasing any detectable amount of reducing sugars, known as amorphogenesis as shown in Figure 2(A).21
Changes of cellulose DP and CrI during enzymatic hydrolysis
A positive correlation between initial hydrolysis rate and CrI for pure cellulosic samples was reported,22 while others have reported that the susceptibility of pre treated heterogeneous lignocellulosic biomass such as eucalyptus chips could not be predicted from the differences in their CrI.23 Amorphous cellulose is generally believed to be hydrolyzed by enzymes at a much faster rate than para-crystalline and crystalline cellulose, therefore it is reasonable to hypothesize that cellulose CrI should increase as the hydrolysis proceeds and this increase could be one of the important reasons causing the gradual slowdown of hydrolysis. Sannigrahi et al.12 found that cellulose CrI increased from 53 to 81% and the relative proportion of para-crystalline and amorphous cellulose decreased after enzymatic hydrolysis of organosolv pre treated Loblolly pine.24 Kafle et al.25 also reported that the CrI of Avicel and bacterial cellulose increased slightly from ~85% to ~90% during the first 12 h of hydrolysis possibility due to the preferential hydrolysis of amorphous cellulose over crystalline part.25 However, their CrI started to decrease after 40% conversion of the total carbohydrate after 12 h of hydrolysis, which implied that cellulose CrI change might not be the major cause for the decrease in hydrolysis rate of these cellulosic samples. Ramos et al. revealed that the hydrolysis of a fully bleached eucalyptus kraft pulp did not appear to result in any significant changes in their CrI.21 In contrast, Betrabet et al. reported that a significant increase in the CrI of substrates as determined by electron diffraction was observed during the hydrolysis of bleached fractions derived from bagasse, wheat straw and hardwood sawdust.26,27 Table 1 summarized the CrI changes during enzymatic hydrolysis of different substrates along with the analytical techniques used to measure CrI in each study. As discussed above and shown in Table 1, it is unclear how cellulose CrI change during hydrolysis up to date and this change strongly depends on the lignocellulosic substrates as well as the mode of action of cellulases obtained from various resources (Penicillium pinophillum vs. T. reesei cellulases). In addition, the cellulose CrI measurement techniques and their impact on interpreting cellulase performance was recently highlighted by Park and co-workers, indicating CrI varied significantly depending on the choice of measurement methods.28
Besides cellulose CrI, changes in the length of cellulose chains or DP were also considered as a factor affecting the rate of enzymatic hydrolysis.23 It is widely reported that hydrolysis usually causes a shift in the molecular weight (MW) distribution of cellulose toward low MW range. Table 2 summarized the effect of enzymatic hydrolysis on DP of cellulose from various cellulosic substrates. Ramos et al. showed a 40% cellulose DP reduction after 10 h of hydrolysis of peroxide pretreated eucalyptus, while 70% of substrate was converted to glucose.21 In contrast, Kafle et al.25 reported the DP of Avicel actually remained constant throughout the entire hydrolysis process, although the hydrolysis activity decreased over time.23 This is possibly due to the fact that Avicel is a crystalline powder and enzyme could only work on the surface of the powders, while deep inside the crystals are not accessible by cellulases. Meng et al.31 studied the change of cellulose DP upon enzymatic hydrolysis of dilute acid (DA) and sodium hydroxide pre treated poplar and switchgrass.31 Their study indicated that the most significant DP variation occurred at the very beginning of the enzymatic hydrolysis and after this initial period, cellulose DP began to decrease at a significantly slower rate. This particular change has been also reported in several other studies.27,32 At the beginning of hydrolysis, this could be explained by the hydrolytic cleavage of internal glycosidc linkages catalyzed by endoglucanase which significantly decreased the cellulose DP. As hydrolysis proceeded, a “peeling-off” mechanism of the newly generated chain ends by exoglucanase action became dominant, leading to a much slower DP decrease.33
Substrates |
Crystallinity index (%) at different hydrolysis time (h) |
Method |
Reference |
|||
Avicel |
~85 (0h) |
~88 (12h) |
~86 (24h) |
~87 (72h) |
XRD1 |
|
Bleached softwood |
~67 (0h) |
~75 (12h) |
~78 (24h) |
~77 (72h) |
XRD |
|
Bacterial cellulose |
~85 (0h) |
~90 (12h) |
~86 (24h) |
~85 (72h) |
XRD |
|
SO2 steam-exploded eucalyptus |
77 (0h) |
76 (2h) |
66 (10h) |
74 (24h) |
XRD |
|
Organosolv pretreated Switchgrass |
52 (0h) |
47 (2h) |
51 (4h) |
53 (8h) |
CP/MAS NMR2 |
|
Microcrystalline Cellulose |
~48 (0h) |
~49 (6h) |
~50 (24h) |
~49 (75h) |
WAXS3 |
|
Table 1 Influence of enzymatic hydrolysis on the CrI of cellulose from various cellulosic substrates.
1XRD: X-ray diffraction
2CP/MAS NMR: Cross-polarization magic angle spinning nuclear magnetic resonance
3WAXS: Wide-angle X-ray scattering
Besides cellulose CrI, changes in the length of cellulose chains or DP were also considered as a factor affecting the rate of enzymatic hydrolysis.23 It is widely reported that hydrolysis usually causes a shift in the molecular weight (MW) distribution of cellulose toward low MW range. Table 2 summarized the effect of enzymatic hydrolysis on DP of cellulose from various cellulosic substrates. Ramos et al. showed a 40% cellulose DP reduction after 10 h of hydrolysis of peroxide pretreated eucalyptus, while 70% of substrate was converted to glucose.21 In contrast, Kafle K, et al.25 reported the DP of Avicel actually remained constant throughout the entire hydrolysis process, although the hydrolysis activity decreased over time.23 This is possibly due to the fact that Avicel is a crystalline powder and enzyme could only work on the surface of the powders, while deep inside the crystals are not accessible by cellulases. Meng X, et al.31 studied the change of cellulose DP upon enzymatic hydrolysis of dilute acid (DA) and sodium hydroxide pre treated poplar and switch grass.31 Their study indicated that the most significant DP variation occurred at the very beginning of the enzymatic hydrolysis and after this initial period, cellulose DP began to decrease at a significantly slower rate. This particular change has been also reported in several other studies.27,32 At the beginning of hydrolysis, this could be explained by the hydrolytic cleavage of internal glucosidic linkages catalyzed by endoglucanase which significantly decreased the cellulose DP. As hydrolysis proceeded, a “peeling-off” mechanism of the newly generated chain ends by exoglucanase action became dominant, leading to a much slower DP decrease.33
Substrates |
Cellulose DP at different hydrolysis time (h) |
Method |
Reference |
|||
Peroxide pretreated eucalyptus |
330 (0h) |
268 (2h) |
246 (5h) |
220 (10h) |
GPC1 |
|
Organosolv pretreated Switchgrass |
2412 (0h) |
1941 (2h) |
2090 (4h) |
2046 (8h) |
GPC |
|
Phosphoric acid-swollen cellulose |
200 (0h) |
80 (4h) |
65 (8 h) |
50 (24h) |
BCA2 |
|
Poplar |
5405 (0h) |
4113 (4h) |
3969 (24 h) |
4034 (72h) |
GPC |
|
Switchgrass |
4639 (0h) |
4079 (4h) |
3390 (24 h) |
3802 (72h) |
GPC |
|
DAP-poplar |
582 (0h) |
155 (4 h) |
219 (24 h) |
231 (72h) |
GPC |
|
Alkaline-poplar |
3950 (0h) |
2776 (4h) |
2201 (24h) |
2124 (72h) |
GPC |
|
DAP-switchgrass |
560 (0h) |
417 (4h) |
325 (24h) |
277 (72h) |
GPC |
|
Alkaline-switchgrass |
3467 (0h) |
2030 (4h) |
1638 (24h) |
1341 (72h) |
GPC |
|
Table 2 Influence of enzymatic hydrolysis on the DP of cellulose from various cellulosic substrates.
1GPC: Gel permeation chromatography
2BCA: DP was determined as the ratio of glucosyl monomer concentration determined by phenol-sulfuric acid method divided by the reducing-end concentration determined by modified 2,2’-bicinchoninate method
Changes of cellulose accessibility and substrate porosity during enzymatic hydrolysis
The accessible surface area of cellulose and substrate porosity is also expected to change during enzymatic hydrolysis. Santa-Maria and co-workers reported the untwisting of cellulose micro fibrils and the appearance of channels along the micro fibril length represented two most important observations of cellulose microstructure change during the early and late stage of hydrolysis, respectively.35 It was also found that a two-step mode of action-an initial fragmentation and a simultaneous swelling and peeling of fragmented fibers was noticed in the hydrolysis of pre treated pulp fibers.36 These cellulose micro and macro-structure changes caused by enzyme attack are suggested as the major reason leading to the changes of cellulose accessibility. Li et al. reported that the volume and surface area of pores smaller than 6 nm decreased after enzyme treatment of cotton fabric.37 The cellulose accessibility of pre treated switch grass and poplar was found to increase at the beginning of hydrolysis and then start to decrease after reaching a maximum value.29 At the beginning of hydrolysis, highly ordered regions of micro fibrils were believed to be delaminated and disrupted known as amorphogenesis, causing the increase of porosity as enzyme further opened up the structure.38 Particle size reduction leading to the increase of external surface area could be another reason causing the increase of cellulose accessibility. Fan et al.39 also showed that the total cellulose surface area of sulfite pulp increased during the first 6 h of enzymatic hydrolysis and then started to decrease likely due to the successive breakdown of pore walls thus facing new challenges.39 On the other hand, a study also showed that a 30% initial decline of specific pore volume was found in the first 2 h of enzymatic hydrolysis of biphasic carbon dioxide and water pretreated mixed hardwoods and switchgrass.40
Lignin and enzyme behaviour during enzymatic hydrolysis
Lignin provides strength, water resistance and protects the plants from pathogens and other environmental factors; however, it also acts as a major barrier for enzymatic hydrolysis of biomass. Lignin inhibition is mainly caused by two ways:
To eliminate this negative effect of lignin, numbers of pretreatment have been introduced to reduce lignin contents or modify lignin structures.42-45 Complete delignification is too costly and excessive lignin removal causes loss of carbohydrates from the biomass; therefore, most of pre treated biomass still contains some amounts of residual lignin and these lignins have different physicochemical characteristics depending on the catalysts, reaction conditions and biomass species.
To better understanding effect of lignin on non-productive enzyme binding, various lignins have been tested.46-52 Table 3 summarizes the adsorption parameters of enzymes onto differently prepared lignins in the recent studies. Adsorption parameters including maximum adsorption capacity, affinity constant and binding strength varies depending on the lignin preparation methods. Pareek et al.40 reported that lignin showed higher adsorption parameters than carbohydrates had.44 In addition, higher enzyme adsorption capacity was observed with the substrates containing lower carboxylic acid groups. Yang and Pan found that functional groups, degree of condensation and hydrophobicity of lignin have strong correlation with adsorption of enzymes onto lignin.46 Hydrophilic modification of lignin by carboxylation and sulfonation reduced lignin inhibition effects by reducing its hydrophobicity. In particular, phenolic hydroxyl group played an important role in the lignin inhibitory effect on enzymatic hydrolysis. Recently, Sun and his colleagues investigated the structural changes of residual lignin by hydrothermal pretreatment on enzymatic hydrolysis.45 They proposed the correlation between condensed syringyl and guaiacyl units and lignin inhibition effect by increasing hydrophobic interaction. Guo et al. found that low S/G ratio and high uniformity of lignin fragment size resulted in high adsorption capacity.53 The adsorption ability of lignin to cellulases depends very much on the nature of the substrates, too. Overall, the impact of lignin chemistry is as significant as that of lignin content in the enzymatic hydrolysis of biomass. Elucidating the changes on physicochemical characteristics of lignin and its enzyme adsorption can suggest solutions for effective enzymatic hydrolysis of biomass.
Biomass species |
Lignin |
Enzymes |
Maximum adsorption capacity [mg/g] |
Affinity [mL/mg] |
Binding strength [mL/g] |
Cottonwood |
lignosulfonate |
cellulase |
127 |
2.9 |
365 |
β-glucosidase |
123 |
3 |
362 |
||
Cottonwood |
alkali lignin |
cellulase |
113 |
2.5 |
282 |
β-glucosidase |
114 |
2 |
232 |
||
Cottonwood |
hydrolytic lignin |
cellulase |
50 |
1.7 |
84 |
β-glucosidase |
44 |
2.1 |
93 |
||
Cottonwood |
organosolv lignin |
cellulase |
94 |
2.3 |
214 |
β-glucosidase |
68 |
2.5 |
169 |
||
Spruce |
Klason lignin |
cellulase |
117 |
1.7 |
195 |
β-glucosidase |
82 |
1.9 |
158 |
||
Poplar |
Klason lignin |
cellulase |
141 |
1.2 |
169 |
β-glucosidase |
91 |
1.3 |
123 |
||
Lodgepole pine |
organosolv lignin |
cellulase |
96-102 |
4-32 |
393-3274 |
Lodgepole pine |
carboxylated lignin |
cellulase |
~19 |
2-4 |
44-74 |
Lodgepole pine |
sulfonated lignin |
cellulase |
14 |
27-30 |
374-415 |
Hybrid poplar |
organosolv lignin |
cellulase |
33-102 |
2-39 |
163-2053 |
Aspen |
milled wood lignin |
cellulase |
2 |
20 |
42 |
Aspen |
hydrothermal pretreated lignin |
cellulase |
3-6 |
13-23 |
58-133 |
Table 3 Adsorption parameters of enzymes for lignins 44-46.
In addition, researches have also been performed to study the adsorption of enzymes directly onto biomass substrate. Table 4 presented enzyme adsorption parameters of a few commonly studied feedstock pre treated by leading pretreatment technologies. The adsorption capacity and binding affinity varies significantly on feedstock type and pretreatment methods employed. Generally, pure cellulosic substrates such as Avicel and filter paper have much less enzyme adsorption capacity than pre treated biomass55 Avicel adsorbed more cellulase than β-glucosidases.54 A recent study using corn stover treated with a few leading pretreatment technologies have revealed that enzyme adsorption is depending on pretreatment methods with relatively higher cellulase adsorption capacity in high pH Pretreatment approaches (AFEX and lime).56 Enzyme related features such as deactivation, inhibition, diffusion constraints, clogging; jamming effects and imperfect processivity might also be responsible for the slowdown of reaction rate.57 It was reported that cellulase can be strongly inhibited by cellobiose, while the adsorption of cellulase on cellulose surface was not affected by the existence of cellobiose.58 Qing et al. added xylan, xylose and various xylooligomers to Avicel hydrolysis and found that xylooligomers were more inhibitory than xylan/xylose and cellobiose/glucose in terms of initial hydrolysis rate decrease.59 Loss of enzymatic activity has also been extensively reported during hydrolysis. Ooshima et al.60 suggested the rapid drop of hydrolysis rate which is always observed in typical hydrolysis of Avicel could be explained by a decline of enzyme activity resulted from a loss of synergistic action between endoglucanase and exoglucanase.60 The exact reasons for deactivation of enzymes due to the enzyme-substrate interaction are still uncertain. One possible mode of deactivation of cellulase could be attributed to shear and interfacial effects, while thermal stability was not found to be a major factor of deactivation.61 Enzyme activity is also expected to decrease due to the formation of concentration gradient caused by diffusion constraints, which could be overcame as the pore size exceed certain limits.9 As the enzyme became trapped inside the small pores, the molecular movement and synergistic interaction could decrease; ultimately lower the enzymatic hydrolysis rate.9 Deactivation of enzymes has been shown to depend on the substrate/enzyme loading and substrate type. For example, it has been reported that the loss of enzyme activity increased as more substrates were introduced during the incubation time and the enzyme-substrate interaction is the main cause of cellulase activity loss which contributes significantly to the slow rate of hydrolysis.62 In addition, a greater decrease in enzyme activity towards filter paper than cotton was also reported.63 Through analyzing the deactivation extents of cellobiohydrolase and endoglucanase, it was suggested that decrease in total cellulase activity was more strongly related to deactivation of cellobiohydrolase than endoglucanase.64 Loss of enzyme activity can be limited by increasing the enzyme loadings. However, Bommarius et al.65 revealed that enzyme molecules binding adjacent to each other onto the small surface area of cellulose, tending to impede one another due to the different rate of bound enzymes proceed at high concentrations of enzymes.65 For example, jamming of adjacent cellulase enzymes when adsorbed on microcrystalline cellulose surface was beyond 400U/L cellulase/8KU/L β-glucosidase. To better understand the complexity of enzymatic hydrolysis reaction, a fractal michaelis kinetics was reformulated by applying the general fractal formalism to the classical model of homogeneous enzymatic reaction coupled to a jamming effect caused by the overcrowding of enzyme in limited spaces.66 Restart enzymatic hydrolysis involving the addition of fresh buffer and enzymes also provides a promising tool to assess if enzyme related factor or lose of substrates reactivity with conversion is responsible for the slowdown of hydrolysis. Several studies have suggested that the hydrolysis rate for restart hydrolysis system was either decreased at a significantly slower rate or relatively stable compared to a normal uninterrupted batch hydrolysis.29,67 These studies strongly suggested cellulose did not actually lose the reactivity as the more easily reacted material are consumed preferentially and the hydrolysis was probably controlled by the amount of enzyme attached to the surface rather than by the changes in substrate surface character.
Pretreatment |
Substrates |
Adsorption method |
Adsorption capacity (mg/g) |
Binding affinity (mL/mg) |
N/A |
Avicel |
4°C, Bio-Rad |
95.2 |
0.3 |
Filter paper |
50°C, Bradford |
86.2 |
- |
|
DAP |
Mixed hardwood |
4°C, Bradford |
80.6 |
1.82 |
Corn stover |
4°C, Nitragen factor |
90.7 |
2.49 |
|
Alkaline |
Corn stover |
4°C, Nitragen factor |
133.6 |
0.88 |
AFEX |
Corn stover |
4°C, Nitragen factor |
99.7 |
1.86 |
SEP |
Pine |
25°C, Ninhydrin |
101.1 |
1.45 |
Corn Stover |
4°C, Nitragen factor |
124.8 |
0.9 |
|
Organosolv |
Pine |
4°C, Bradford |
35.1 |
3.11 |
Sweetgum |
4°C, Bradford |
60.2 |
1.31 |
Table 4 Adsorption parameters of enzymes for untreated and pretreated substrates.
N/A: not applicable; DAP: dilute acid pretreatment; AFEX: ammonia fiber explosion;
SEP: steam explosion pretreatment; -: not reported.
This review focuses on understanding the limitations occurring during enzymatic hydrolysis of lignocellulosic substrates that might be responsible for the gradual slowing down of the reaction. Substrates characteristics such as cellulose DP, CrI, accessibility and enzyme related features including enzyme inactivation and inhibition are all suggested to be responsible and the relative contributions of each factor depend strongly on the substrates, the type of enzyme and pretreatment conditions. Long enzymatic hydrolysis time and high enzyme loading add significant operating costs to the overall biomass to ethanol bioconversion process. Analyzing how the biomass structural relevant factors change during enzymatic hydrolysis is essential for strengthening the understanding of intrinsic hydrolysis reaction mechanisms and interactions between cellulase enzymes and plant cell wall components, therefore ultimately reducing the costs associated with enzymatic hydrolysis.
The author declares no conflict of interest.

©2016 Meng, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.