Journal of
eISSN: 2572-8466


Research Article Volume 6 Issue 4
Department of Biological Engineering, Utah State University, USA
Correspondence: Foster A Agblevor, USTAR Bioenergy Center, Department of Biological Engineering,Utah State University, Logan UT 84322, USA
Received: August 10, 2019 | Published: July 26, 2019
Citation: Agblevor FA, Diallo O. Improvement in cell growth and ethanol productivity of Saccharomyces cerevisiae due to poultry litter biochar. J Appl Biotechnol Bioeng. 2019;6(4):179-186. DOI: 10.15406/jabb.2019.06.00191
The effect of poultry litter biochar on Saccharomyces cerevisiae growth and ethanol fermentation was investigated for glucose, steam exploded corn stover and poplar wood. S. cerevisiae grew faster in both anaerobic and aerobic biochar medium than the control media. For the same initial concentration of glucose, the biochar medium glucose was consumed in 12 hours compared to 24 hours for control and the maximum ethanol productivity were higher for the biochar compared to the control medium. When the initial glucose concentration was increased the maximum ethanol productivity for the biochar media was more than two times that for the control. Similarly, addition of poultry litter biochar to steam exploded poplar wood and corn stover improved the ethanol productivity depending on the amount of biochar added to the fermentation medium. Thus, biochar is an effective additive to reduce ethanol fermentation time.
Keywords: Saccharomyces cerevisiae, biochar media, black solid, ethanol
Poultry litter biomass is a mixture of bedding material, manure, feathers, spilled feed, which could be potentially used as feedstock for biofuel production. Poultry litter biochar is the black solid residue obtained in addition to bio-oil and gases after pyrolysis of poultry litter biomass.1 Biochar has three important physical properties: aromatic structure, porous structure, and pore volumes.2 The physical properties and the composition of biochar depend on the feedstock and the pyrolysis conditions.3 Biochar have been the focus of several studies due to its potential uses and applications in different domain, and its impact on microbial growth, soil improvement, and plant growth.4,5 The application of biochar to soil has received much attention due to its high stability in soil and its potential to mitigate soil-derived greenhouse gas emissions. This was shown by the study of the Terra Preta soils in the Amazonian rainforest6 and by the study conducted by Lehmann et al.7
The impact of biochar on microbial community’s structure and function have been reported by several researchers.5,8–11 These researchers reported that mycorrhizal biomass was increased when biochar was added to the soil and this was attributed to the availability of extra nutrients from the biochar. The presence of the biochar also affected the distribution of the microbial community. Some researchers reported that the ratio of Gram+ to Gram- increased with the addition of biochar during composting.5 The presence of fertilizer also influenced the effect of biochar; when fertilizer was present the biochar effect was significant, but in the absence of fertilizer, there was no significant effect of biochar.10 Biochar was reported to increase soil microbial biomass, root nodulation by rhizobia, and arbuscular mycorrhizal fungi.2,11 The obvious positive attribute of biochar is more associated with its physical properties and nutrient value. Jindo et al.,5 reported that biochar can affect soil microbial community through their high porosity which provide adequate microhabitat for microorganisms in the soil. However, the exact effect of biochar on the microorganisms is still unclear.2 Investigations of anaerobic digestion (AD) for the production of biomethane showed that biochar can have some influence on the methane production. The presence of biochar could stabilize the AD medium, it could adsorb some inhibitors and thus improve on the digestion and it could also act as a buffer during AD.
Poultry litter biochar contains nutrients such as: nitrogen, phosphorous, potassium, calcium, magnesium, iron, and sodium.1,3,12 These minerals were reported favorable for biological uptake for microorganisms and also soil food web.13 Saccharomyces cerevisiae is an important species used in the alcohol fermentation under anaerobic condition. The major constituents of culture media for yeast growth are: carbon source (e.g. sugars), nitrogen source such as organic (e.g. peptone, yeast extract) and inorganic (ammonium salts, nitrate nitrogen), and minerals (e.g. K, Ca, Na, Mg, and trace elements). Some of these minerals are present in poultry litter biochar and could be utilized by the fermenting microorganism for growth and product formation if they are bioavailable.1,14 The goal of this research is to use poultry litter biochar to improve the hydrolysis and fermentation of pretreated lignocellulosic biomass for bioethanol and other bioproducts production. To the best of knowledge of the authors, biochar has not been used as additive for the fermentation of ethanol. In this paper we report the results of S. cerevisiae cell growth and fermentation of glucose and pretreated biomass in the presence of poultry litter biochar.
The yeast strain used in this study was S. cerevisiae ATCC 204508/S288C. Poultry litter biochar (PLB) was produced at the USTAR Bioenergy Center, Utah State University. The poultry litter biomass was supplied by Virginia poultry farmer in Shenandoah Valley, Virginia.1 The moisture, ash content, and elemental composition of the biochar were determined as described below.
Poultry litter biochar characterization
The poultry litter biomass was ground to pass 2-mm mesh and stored at room temperature until time of pyrolysis. The pyrolysis was conducted at 500°C and 2 seconds residence time.1,15 The recovered biochar was characterized, stored, and used for the studies described below. Additionally, pine wood was also pyrolyzed at 500°C and 2 seconds residence for comparison with the PLB.
The moisture content of PLB was determined using an infrared moisture analyzer (Denver Instrument IR-60, Bohemia, NY). About 1.00g of PLB sample was weighed into a tared sample pan and its moisture was determined by heating at 105°C for 30 minutes. At the end of the 30 minutes, the moisture content was automatically calculated and displayed. The ash content of PLB was determined using ASTM E1755-01 (Reapproved 2007) standard method: samples were prepared in triplicate. About 1.0g of each sample was weighed into a pre-weighed porcelain crucible and ashed at 575°C for 8 hours using a muffler furnace (Lindbergh Blue, Thermo Scientific, Asheville, NC). The ashed samples were weighed until a constant mass was achieved, and the ash content was calculated on moisture-free basis. All samples were determined in triplicates.
The elemental composition (C, H, N, S, O) of the PLB were determined using a Flash 2000 organic elemental analyzer (CE Elantech Inc, Thermo Scientific, Lakewood, NJ). The Brunauer-Emmett-Teller (BET) specific surface area of PLB was measured using a Quanta Chrome Monosorb instrument (model MS-16 Quanta chrome, Syosset, NY) through nitrogen adsorption. The Quanta Chrome Monosorb was calibrated with air before the surface analysis. All samples were determined in triplicates and were out-gassed for 3h at 220°C before BET surface measurement.
Fermentation materials
S. cerevisiae was cultivated in biochar, yeast malt (YM), and GYE (glucose+yeast extract) media. For the liquid media preparation, YM broth powder and glucose (D-(+) glucose) were purchased from Sigma Aldrich (Sigma Aldrich, St Louis, MO), Bacto™ yeast extract was purchased from Fischer Scientific (Pittsburgh, PA). Yeast cells were grown in 250ml Erlenmeyer flasks, duplicate flasks were prepared. Solid media were also prepared for colonies counting method.
YM media
The YM media were prepared the same throughout the study, approximately 2.1g of YM powder was weighed and added to 250ml flask, 100 ml of deionized water (di-water) was added to dissolve the powder, and the pH was 6.3. The mixture was autoclaved at 121°C for 30 minutes, and left in the laminar hood to cool to room temperature.
Biochar media
The biochar media were prepared using glucose, PLB, and yeast extract. Two 250ml Erlenmeyer flasks were used to prepare each biochar medium; glucose, biochar, and 50ml of di-water were added to the first flask. Yeast extract and 50ml of di-water were added to the second flask. The two flasks were sterilized separately to avoid Maillard reaction between the glucose and the proteins in the yeast extract which will result in the loss of nutrients. The flasks were autoclaved at 121°C for 30 minutes and left in the laminar hood to cool to room temperature. The yeast extract solution and glucose and biochar mixture were mixed to form the biochar medium. Before inoculation the pH was adjusted to 6.3 using 1M HCl.
GYE media
The GYE media were prepared using glucose and yeast extract only, the glucose and yeast extract were prepared separately using two different flasks. Glucose and 50 ml of di-water were added to the first flask. Yeast extract and 50ml of di-water were added to the second flask. The flasks were autoclaved at 121°C for 30 minutes and left in the laminar hood to cool to room temperature. The yeast extract and glucose solutions were mixed to form the GYE medium. Before inoculation the pH was adjusted to 6.3 using 1M HCl.
Steam exploded biomass
The poplar wood and corn stover steam exploded biomass were prepared using a bench scale autoclave steam exploder at Virginia Tech. The steam explosion temperature was 210°C and the residence time was 2 min. After steam explosion poplar wood material was first washed with distilled water before being dried at laboratory temperature and then stored in the fridge until the time of fermentation. In the case of the corn stover, there was no washing of the steam exploded material. This material was dried at laboratory conditions like the poplar wood and then stored in freezer bags in the fridge until the time of hydrolysis and fermentation studies.
Hydrolysis of steam exploded materials
The stem exploded materials were subjected to separate hydrolysis and fermentation process. This method was selected in order to determine the effect of the biochar on the enzyme hydrolysis process. Poultry litter biochar was added to the steam exploded poplar (SEP) and hydrolyzed using CTec2 enzyme supplied by Novozymes (Novozymes, Franklinton, NC). 10g of the SEP was added to screw cup flasks, and biochar (0.0g, 0.2g, 0.5g, 1g, 5g, and 10g) were added to each flask. 100ml of citrate buffer pH: 4.8 was added to each flask, and flasks were autoclaved at 121°C for 30 minutes. The flasks were cooled to room temperature under laminar hood, and CTec2 was added to each flask at a loading of 17FPU/g (g of biomass dry matter). The flasks were then incubated at 50°C and 130 rpm in a water bath shaker incubator (Precision Scientific, East Lyme, CT) for 48 hours.
The steam exploded corn stover (SECS) was hydrolyzed with and without biochar addition at the similar conditions as the SEP. The biomass loading was 10 wt.%, biochar loadings were 0.2 wt.% and 1 wt.% and the CTec2 loading was 10FPU/g.
Inoculum preparation
The S. cerevisiae cells were cultivated aerobically in YPD (Yeast Peptone Dextrose) medium (20g/l glucose, 20g/l peptone, 10g/l yeast extract) for 16 hours in a shaker at 37°C and 220 rpm, and used to inoculate the YM, biochar, and GYE media. The optical density of the inoculum cultures varied between 5.1 and 6.8, and each time about 82.4mg dry weight of yeast was aseptically added to each flask.
Culture growth and measurement methods
The cultures were incubated in an incubator-shaker (MaxQ 6000, Thermo Scientific, Asheville, NC) at 37°C and under shaking at 220 rpm for 24 hours. Batch cultivation was performed under aerobic and anaerobic conditions. For the aerobic growth, foam stoppers and aluminum foils were used to plug the flasks to allow breathing. Aliquots were taken every 3 hours to measure the optical density and to count colonies.
Fermentation
For the fermentation process the separate hydrolysis and fermentation (SHF) method was used in order to determine the independent effect of the poultry litter biochar on the hydrolysis of the steam exploded material as well as the fermentation. Poultry litter biochar was added to steam exploded biomass (poplar and corn stover) and hydrolyzed using CTec2 enzyme as described above. Glucose and steam exploded hydrolysates (SEP and SECS) fermentation was carried out using S. cerevisiae ATCC 204508/S288C. The inoculum was cultivated using YPD medium for 16 hours, at 37°C and 220 rpm. The cells were harvested after 16 hours; the optical density at 600nm was 6.16. The cells were centrifuged at 3000xg for 10 min under sterile condition; the supernatant was discarded, and the cells were transferred to 250 ml screw-capped Erlenmeyer flasks containing the hydrolysates. Flasks were purged with nitrogen for 5min then capped to allow fermentation but not completely tight in order to allow the CO2 to escape. The samples were fermented at 37°C and 130 rpm in a water bath incubator shaker. Fermentation samples were taken and analyzed to determine cell mass, glucose, and ethanol contents.
Glucose analysis
During the 150g/l glucose fermentation, the glucose consumption was measured by the dinitrosalicylic acid (DNS) method16 and for the 50g/l glucose and the steam exploded biomass (poplar and corn stover), the glucose was measured by high performance liquid chromatography (HPLC) method. The hydrolysate samples were filtered through 0.2µm nylon membranes and analyzed to determine the sugar content using HPLC method. The HPLC (LC-10AT Shimadzu) was equipped with evaporative light scattering detector (ELSD), an auto sampler (SIL-Shimadzu), and a Prevail™ carbohydrate column (250mmx4.6mm). The column temperature was 30°C, the detector temperature and pressure were 50°C and 350 kPa respectively. The mobile phase composition was 20/80 water/acetonitrile, and the flow rate was 1 ml/min.
Ethanol extraction
At the end of the fermentation, the broth was centrifuged to separate the biomass and the cells from the liquid broth. Before injecting the samples into the GC the organic phase was extracted from the liquid broth to avoid introducing sugars into the GC column. 500µl of the fermentation broth was added to a centrifuge tube, and 5µl of n-propanol was added as an internal standard. The mixture was vortexed for 30seconds, then (0.5mlx3) of Methyl Isobutyl Ketone (MIBK) was added; the mixture was again vortexed for 5 minutes. Finally, all tubes were centrifuged for 5 minutes at 3000xg to facilitate phase separation. The organic phase (upper layer) was recovered and manually injected into GC for ethanol analysis. Analysis of ethanol and n-propanol was performed using HP 6890 Series GC, equipped with an Agilent capillary column (HP-5, 30m x 0.320 mm x1.00µm id), and a flame ionization detector (FID). 1µl of the upper layer of the extract was injected at the following conditions: the detector and inlet temperatures were 300°C, and 250°C respectively, the oven was programmed at initial temperature of 40°C, and held for 1.7 minute; the ramp was 15°C /minute, and the final temperature was 250°C and held for 2.67 minutes.
Poultry litter biochar characterization
Table 1 shows the composition of the poultry litter biochar (PLB); the ash content was 55.3±0.25 %, carbon content was 23.4±1.34 %, and nitrogen content was 2.3±0.09 %. These compositional data were similar to the composition of PLB reported.1,14 In general PLB had low carbon, higher nitrogen and ash contents compared to woody biochar.12,17 A typical composition of pine biochar is shown in the Table 1 for comparison. Poultry litter biomass was reported to contain high inorganic elements and low carbon compared to woody biomass.15 The oxygen content determined by difference was 14.74±1.37 %. In this study, chlorine was not determined, and the oxygen content may also include chlorine. The BET specific surface area was low (6.34±0.19m2/g) because the PLB was produced at 500°C. Biochar produced at 500°C and below was reported to have extremely low surface area.18
|
|
|
Elemental Analysis of poultry litter biochar (wt. %) |
|
||||||||
Sample |
Moisture (%) |
Ash (%) |
C |
H |
N |
S |
Oa |
SSA (g/m2)b |
||||
1 |
2.9 |
55.49 |
22.21 |
1.03 |
2.26 |
0.55 |
15.55 |
6.56 |
||||
2 |
2.71 |
55.01 |
23.17 |
1.07 |
2.3 |
0.23 |
15.51 |
6.23 |
||||
3 |
2.88 |
55.32 |
24.85 |
1.15 |
2.44 |
0.2 |
13.16 |
6.23 |
||||
Average |
2.83±0.10 |
55.27±0.25 |
23.41±1.34 |
1.09±0.06 |
2.33±0.09 |
0.33±0.19 |
14.74±1.37 |
6.34±0.19 |
||||
Elemental analysis of pine biochar wt%) |
||||||||||||
2.71 |
10.35 |
70.36 |
2.77 |
0.51 |
0.00 |
13.40 |
||||||
Table 1 Composition of poultry litter biochar and pine biochar
aO by difference, bspecific surface area.
Aerobic growth of S. cerevisiae
The optical density was measured at 600 nm and plotted against time to represent the growth curves. In Figure 1A, the growth curves show that the yeast growth in the PLB medium was about two times higher than the controls and was statistically different from the growth in the control media, YM medium (p-value=0.0245<0.05) and GYE medium (p-value=0.0283<0.05), indicating that addition of PLB had a positive effect on the yeast growth. However, there was no difference in the growth curve of the pine wood biochar and the controls showing that the biochar by itself devoid of nutrient had no influence on the growth of the yeast cells.
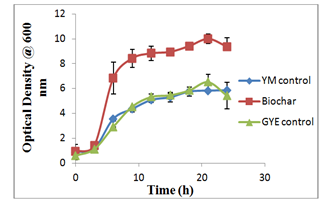
Figure 1A cerevisiae growth in YM medium (21g/l), Biochar medium (20g/l glucose, 10g/l yeast extract, 2 g/l PLB), GYE medium (20g/l glucose, 10g/l yeast extract.
Chicken manure biochar amended soil was reported to increase microbial activity, and population growth capacity of the microbial community.19 Biochar addition during composting of tomato stalk and chicken manure had influence on bacterial community.20 In this study, the growth of S. cerevisiae increased when PLB was added to the medium. Steiner et al.,13 reported a similar effect in their study (in a highly weathered Amazonian upland soil), when glucose was added to soil amended with biochar and water, soil microbial activity increased greatly. Thus the effect reported by these authors are in agreement with our observation on the growth of the yeast cells. The yeast colonies were counted to corroborate the results obtained from the spectrophotometric method. In Figure 1B the growth curves show that the PLB medium had more colonies than the YM and GYE medium, which is similar to the trend observed in Figure 1A. The number of CFU/ml at the exponential phase of the PLB growth curve was higher and significantly different from the YM growth curve (p-value=0.0035<0.05) as well as the GYE growth curve (p-value=0.0085<0.05).
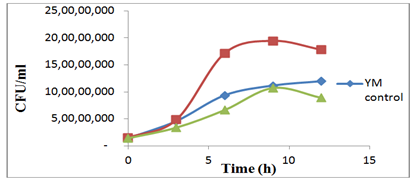
Figure 1B S. cerevisiae colonies in YM medium (21g/l), Biochar medium (20g/l glucose, 10g/l yeast extract, 2 g/l PLB), GYE medium (20g/l glucose, 10g/l yeast extract).
The microscopic slides (Figure 2) show S. cerevisiae in YM medium (21g/l), PLB medium (20g/l glucose, 10g/l yeast extract, 2g/l PLB), and GYE medium (20g/l glucose, 10g/l yeast extract) cultivated aerobically for 9 hours. The slides confirmed that PLB did not inhibit the growth of S. cerevisiae; the cells were able to divide and multiply in the presence of PLB in the medium. In Figure 2A, the slide show that the yeast grown in the PLB medium after 9 hours cultivation had about twice as many colonies as the yeast grown in the YM and GYE media (Figures 2 (B &C)) for the same length of time. When pine wood biochar that contained minimal nutrient was use to cultivate the cells, there was no difference between the controls and the pine wood biochar.

Figure 2 Microscopic slides of S. cerevisiae aerobic growth at 9 hours in biochar and controls media (a) biochar, (b) YM control, and (c) GYE control.
Glucose fermentation
The cell growth pattern under anaerobic conditions was similar to those observed for the aerobic conditions. PLB medium had higher cell mass than the control GYE medium (p-value=0.0329< 0.05). The glucose was completely depleted in 12 hours in the PLB medium, while less than a half of the glucose in the GYE medium was consumed in 12 hours, and it took at least 24 hours for the glucose in the GYE medium to be completely consumed. The ethanol production was faster in the PLB media compared to the control GYE media. The ethanol concentrations were 13.45g/l in the PLB medium and 2.89g/l in the GYE medium at 6 hours when the initial glucose concentration was 50g/l. In the PLB medium, the maximum ethanol was produced at 9 hours, and was 15.46g/l, and then the ethanol started decreasing because the glucose was completely consumed. Whereas, in the GYE medium the maximum ethanol was 16.46g/l at 9 hours and it stabilized because more than half of the glucose remained in the medium. The data showed that glucose was consumed faster in the PLB medium than that in the control GYE medium obviously because of the higher cell mass in the PLB medium.
When the initial glucose concentration was increased to 150g/l, the cell mass concentration in the PLB medium was near the maximum optical density (OD) within twelve hours of fermentation and was more than twice (2.5x) that in the control medium (Figure 3). It can be seen from Figure 3 that the slope of the PLB medium growth curve was very steep compared to the control medium. Cell growth in the control medium was sluggish and continued to increase up to 48 hours whereas with 24 hours the PLB medium was maximum and then started to decrease slightly probably due to autolysis. The sluggish growth of the control medium was probably due to substrate inhibition. In case of glucose fermentation, it has been reported that above 175g/l, the yeast was not capable of fermenting the sugar because of osmotic stress and the production of some unknown inhibitory compounds in the fermentation broth.21 In addition, ethanol has been shown to inhibit the fermenation pathway for ethanol production when the ethanol concentration was above 55g/l for some yeast species.22 The poor performance of the control in the current system is akin to osmotic stress reported in literature.23 The current data clearly showed that, at 150g/l initial glucose concentration, the presence of PLB appeared to reduce osmotic stress and increased cell mass production and therefore ethanol concentration increased.
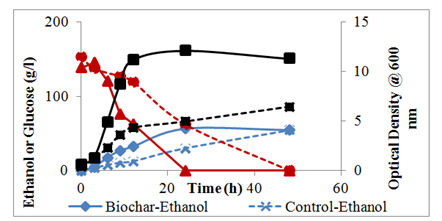
Figure 3 S. cerevisiae growth under anaerobic condition in the GYE control (150g/l glucose, 10g/l yeast extract), and biochar medium (150g/l glucose, 10g/l yeast extract, 2g/l biochar).
The glucose in the PLB medium was completely depleted within 24 hours whereas it took up to 48 hours before the control glucose was depleted (Figure 3). At 24 hours fermentation time only 56% of the glucose was consumed in the control medium. These data corroborate the OD data in that the higher the cell density, the more rapid the glucose would be consumed. The maximum ethanol concentration in the PLB medium occurred at 24 hours and was 56.3g/l compared to 30.1g/l in the control at the same time (Figure 3). The slow ethanol production in the GYE control medium was attributed to substrate inhibition; however, in the presence of PLB, there appeared to be less substrate inhibition and ethanol was produced at a faster rate. During the fermentation process the flasks with the PLB media started to foam early but the flasks with the control GYE media did not have any foam during the early fermentation hours. At 9 hours fermentation, flasks with the PLB media all had a lot of bubbles; whereas the flasks with the control GYE media did not have any bubbles indicating that the fermentation was faster in the PLB media compared to the control GYE media. Since ethanol production is growth associated, it implies that with faster cell mass production in the PLB medium, ethanol production will be faster than in the control medium.
Yeast extract is a source of trace elements, vitamins, and amino acids for the yeast growth. S. cerevisiae was cultivated in the PLB and glucose medium in the absence of yeast extract in order to determine whether PLB was a sufficient source of nutrient. The data showed that the growth curve of the yeast in the YM medium was higher than that of yeast in the PLB (PLB only) medium, which indicated that the nitrogen in the PLB may not be bioavailable for the yeast uptake. Lang et al.,24 & Zheng et al.,18 reported that the nitrogen (N) was lost during high temperature pyrolysis, and the decrease in available N in PLB begun at 400°C during pyrolysis. DeLuca et al.,25 also reported that N was associated with many organic molecules and it started to volatilize at 200°C.
Addition of an alternative source of nitrogen was necessary for poultry litter biochar to stimulate the yeast growth. Revell et al.,14 reported that addition of nitrogen fertilizer to biochar increased pepper yield in the sandy loam soil. The elemental composition of yeast extract was performed and the N content was 10.29±0.21 %. A similar amount of N was added to the PLB medium by substituting the yeast extract with ammonium sulfate. The addition of (NH4)2SO4 to the medium improved the yeast growth compared to the growth in (PLB only) medium and the growth curves were statistically different (p-value=0.0405<0.05). However, the yeast growth in (PLB+(NH4)2SO4) medium was less than the yeast growth in the YM medium. This suggested that other components in yeast extract such as amino acids that contributed to the cell growth but were deficient in the poultry litter biochar. When the amount of yeast extract added to PLB medium was varied from 1.0g/l to 10.0g/l, the growth rate of the cells varied accordingly with the optimum being 10g/l, which is normally used for most yeast fermentations.
Steam exploded poplar wood (SEP)
The glucose concentrations after separate enzyme hydrolysis (SH) for control sample (0% PLB) and samples with PLB concentations of 0.2 % , 1 %, and 5 % were 45.51g/l, 43.61g/l, 45.95g/l, and 38.41g/l respectively. During the separate hydrolysis and fermentation (SHF), the glucose was consumed faster by the yeast when PLB was present and the glucose consumption increased as the PLB concentration was increased. The maxium ethanol was produced at 6 hours for the sample with highest PLB loading (5 %); at 12 hours for the samples with lower PLB loadings (0.2 %, and 1 %); and 24 hours for the control sample. The maximum ethanol concentration for the control, 0.2%, 1%, and 5% PLB were 23.6g/l, 22.3g/l, 23.2g/l, and 19.22g/l respectively. The ethanol concentration at the highest PLB loading was lower than that of the control because the cells utilized some of the ethanol after the glucose was depleted within 6 hours.
Steam exploded corn stover (SECS)
PLB was also added to SECS during the SHF and the glucose and ethanol concentrations were measured during the fermentation and compared with the control sample (0 % PLB). The glucose concentrations after separate enzyme hydrolysis were 29.09g/l for the control, 34.96g/l for 0.2 % PLB, and 33.64g/l for 1% PLB (Figure 4). During SHF, the glucose consumption by the yeast was faster in the PLB samples than the control sample, which was similar to that observed for the SEP. Glucose was almost completely consumed within 9 hours in 1 % PLB sample; 12 hours in 0.2 % PLB sample; and 24 hours in the control sample respectively (Figure 4). The slower glucose consumption in the control sample was attributed to the presence of inhibitors such as furan, acetic acid or formic acid in the untreated sample. PLB addition to the SHF of SECS improved the ethanol production; the maxium ethanol concentrations were achieved within 12 hours for the samples with 1 % and 0.2 % PLB loadings with respective ethanol concentrations of 19g/l and 16.7g/l. For the control sample, the ethanol did not reach the maxium concentration until the glucose was completely consumed at 24 hours (Figure 4). Thus, it appeared that the PLB either adsorbed some of the inhibitors or the high cell concentrations reduced the toxic effects of the inhibitors and hence the better formance of the yeast in the PLB medium.
Glucose
Addition of PLB improved the ethanol productivity for the glucose fermentation. When the initial glucose was 50g/l the maximum ethanol productivity was 2.17g/l-h for PLB medium and 1.78g/l-h for the GYE control with no PLB. Similarly when the initial glucose concentration was increased to 100g/l and 150g/l, the maximum ethanol productivities were 3.76g/l-h and 2.99g/l-h respectively for the PLB media, and the corresponding values for the control were 1.24g/l-h and 1.19g/l-h respectively (Figure 5). The highest ethanol productivity (3.76g/h.l) was obtained with the PLB media when the initial glucose concentration was 100g/l and the lowest ethanol productivity was obtained with the GYE control media when the initial glucose concentration was 150g/l-h (Figure 5). Notice that for the PLB media, when the initial glucose concentration was increased from 50g/l to 100g/l, the maximum ethanol productivity increased as well. The GYE control media showed a similar productivity trend when the initial glucose concentration was 100g/l and 150g/l, the lower ethanol productivity of the control was attributed to substrate and ethanol inhibitions.
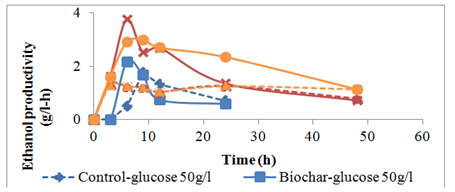
Figure 5 Ethanol productivity by S. cerevisiae from glucose fermentation for controls (no biochar) and 0.2% biochar addition.
Steam exploded biomass
As observed for the pure glucose fermentation, addition of PLB also had a positive effect on the ethanol productivity SHF of the steam exploded biomass (Figure 6). For the SEP, the maximum ethanol productivity increased as the PLB loadings increased from control (no PLB), 0.2%, 1 %, and 5% PLB and were 1.68g/l-h, 1.94g/l-h, 2.26g/l-h and 3.2g/l-h respectively. The sample with 5% PLB loading had the highest productivity (3.2g/l-hr) while the control had the lowest (1.68g/l-hr) (Figure 6).
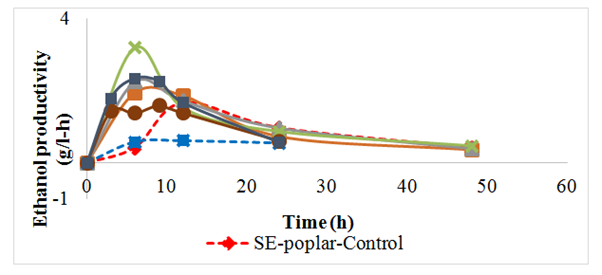
Figure 6 Ethanol productivity by S. cerevisiae from steam exploded poplar (SE) poplar and corn stover for controls (no biochar) and biochar addition.
Similarily, for the SECS hydrolysates, the maximum ethanol productivity increased as the PLB loadings were increased and were 0.62g/l-h for control, 1.6g/l-h for 0.2% PLB, and 2.02g/l-h for 1% PLB (Figure 6).
The maximum ethanol productivities obtained for the 1% PLB loading in the case of SEP and SECS were 2.26g/l-h and 2.02g/l-h respectively, and they both occurred at the same time (6 hours). However, the maximum ethanol productivity for 0.2 % PLB loading occurred at different times and were 1.6g/l-h at 9 hours for SECS versus 1.94g/l-h at 6 hours for SEP (Figure 6). The shorter ethanol production time observed in the case of SEP may be due to the fact that the biomass was washed with water after steam explosion pretreatment while the SECS was not washed. Studies have shown that washing the biomass with water after steam explosion pretreatment removed some inhibitory compounds,26 which can explain the overall increase in ethanol production from poplar compared to corn stover.
The role of poultry litter biochar in cell growth and fermentation of ethanol
The addition of PLB to both the growth and fermentation media clearly increased cell mass for aerobic and anaerobic conditions. In most cases the cell mass was about twice that of the control sample. The cells were also able to grow in relatively high initial glucose concentration medium without much substrate inhibition. These observations suggest that the PLB played four roles in the fermentation and cell growth processes: particulate effect, nutrient effect, osmotic effect, and absorption of inhibitory compounds. Two experiments were conducted with the PLB, in one case the the mixture of glucose and PLB was filtered after sterilization (at 121°C for 30min) to remove the PLB particles while the other sample was not filtered. When the fermentation was conducted, the medium with the PLB particles grew faster and produced ethanol at a faster rate than the filtered medium. This suggested that besides the nutrient in the PLB, the particles also played some role in the cell growth and ethanol fermentation. Iconomou et al.,27 reported that the addition of γ-alumina to the molasses fermentation medium improved the ethanol production but did not affect the cell growth of S. cerevisiae. Groat & Ough28 added bentonite and other insoluble solids to clarified grape juice and observed faster and more complete fermentation than the control, but they did not report the effect on cell mass concentration. Kuhbeck et al.,29 investigated the effect of hot trub, kieselguhr, and poly(vinylpyrroldones) particles on the performance of S. cerevisiae during fermentation of wort and reported that there was cell mass increases of 20-50% depending on the particle size distrbution as well as the nutrient value of the particle. Addition of activated carbon,30 Chromosorb31 to wort medium were also reported to have stimulatory effect on yeast cell and cell mass increased significantly during fermentation. However, all these wort fermentations were conducted at very low temperatures (<20°C) where yeast cells were reported to be more tolerant to ethanol and solubility of CO2 is relatively high.32 The inert particulates were speculated to form the center of nucleation of CO2 and thus reducing the CO2 concentration in the wort. CO2 is known to be inhibitory to microbial growth beyond some concentration levels.32 In the present studies, fermentations were conducted at 37°C where the solubility of CO2 is relatively low and therefore the growth stimulation observed cannot be attributed to CO2 nucleation and removal to reduce toxicity.33–35
The influence of the PLB on the fermentation could also be due to adsorption of both the glucose and ethanol on the PLB; thus 200g/l glucose solution in water was prepared and 2g/l PLB was added and stirred for 48 hours. Aliquotes were taken at various time intervals and analyzed. The results showed that there was no statistically significant adsorption of the glucose over time. A similar mixture was prepared with 100g/l ethanol and 2g/l PLB and stirred continuosly for 48 hours. In this case also, the concentration of the ethanol did not change with time. In both cases no compound was adsorbed, so it was concluded that the adsorption of substrate and product did not play any significant role on the performance of the PLB during the fermentation process. The PLB also contain soluble salts of zinc, magnesium, potassium, iron etc,1 which could serve as nutrient for the yeast. However, in the absence of yeast extract these salts had no stimulatory effect, infact in some cases they supressed cell growth. Revelle et al.,14 reported that PLB suppressed pepper plant growth if the concentrations exceeded some threshold level because of salt toxicity effect; they had best results when the PLB was supplemented with nitrogen fertilizer. In the absence of the biochar particulates cell growth was lower than those with particulates (data not shown).
When high initial glucose concentrations were fermented with PLB present at a concentration of 2g/l, there appeared to be minimal inhibition compared to the control. However, addition of glucose to PLB showed that there was no statistically significant adsorption of the glucose on the PLB. Thus, the PLB appeared to moderate the effect of substrate inhibition without adsorbing it. The the addition of the PLB to the steam exploded biomass also showed that the reaction was faster than the control without PLB. This suggests that the PLB particles either adsorbed some of the toxicants or moderated their toxic effect on the S. cerevisiae cells.
In general the amount of cell mass produced was much higher in the PLB medium than the control and was aided by yeast extract, suggesting that the effect observed for PLB was not just due to the particulate matter, but perhaps was a synergistic effect of particulate matter, soluble inorganic nutrients, and amino acids in yeast extract. World bioethanol production according to the Renewable Fuel Association (RFA) was 28570 million gallons in 2018 of which the USA and Brazil produced 16061 and 7920 million gallons respectively.36 The ethanol is produced mostly from corn (USA) and sugarcane (Brazil) using traditional yeast fermentation; the contribution from cellulosic ethanol is insignificant. The traditional batch fermentation time of glucose to ethanol either from starch or sugarcane is about 2-3days to reach acceptable levels of ethanol concentration for subsequent distillation. Most yeast strains cannot tolerate ethanol concentration higher than 10%, which implies distillation of high volumes of water to obtain pure ethanol. The addition of PLB improves the overal ethanol production process in that, the fermentation time is almost halved and therefore twice as much ethanol can be produced for a given time period. Secondly, the PLB improves alcohol tolerance and reduces substrate inhibition and hence higher level of alcohol can be produced and this will reduce distillation cost. Thus, the addition of PLB to the fermentation media will have major positive effects on large-scale bioethanol production.
Poultry litter biochar (PLB) contains a host of inorganic elements such as Mg, Ca, K that has a strong influence on cell growth and fermentation of ethanol by S. cerevisiae. The addition of PLB to S. cerevisiae aerobic and anaerobic media promoted the rapid growth of the cells to about two times that of the control. Because of the rapid increase in cell mass, the ethanol fermentation times were reduced to almost half that of the control and the ethanol productivity was very high for the PLB medium. Yeast extract was essential for the effectiveness of PLB on the cell growth and fermentation of ethanol. In the absence of yeast extract, the effect of PLB was not significant on both cell growth and ethanol production. The presence of PLB in the medium appeared to reduce substrate inhibition and therefore higher initial glucose concentration was fermented to relatively high ethanol concentration in the fermentation broth. About 2.0g/l PLB was sufficient for rapid cell growth and fermentation. The stimulatory effect of the PLB was effective for both glucose and steam exploded biomass hydrolysates. This new method can be potentially used to reduce ethanol fermentation times and have major impact on the bioethanol industry.
The authors acknowledge Dr Timothy Taylor for providing the yeast samples for these studies. Robert Wright, Virginia Polytechnic Institute and State University, Blacksburg, VA is acknowledged for providing the steam exploded biomass samples. The Utah Science Utah Science Technology and Research (USTAR) Program is acknowledged for funding the research.
The author declares there are no conflicts of interest.

©2019 Agblevor, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.