Journal of
eISSN: 2572-8466


Research Article Volume 11 Issue 4
1Environmental Microbiology Laboratory
2Signal Transduction Laboratory
Correspondence: Juan Manuel Sanchez-Yañez Chemical Biological Research Institute, Ed-B3, University City. Universidad Michoacana de San Nicolas de Hidalgo, Francisco J. Mújica S/N,Col Felicitas del Rio ZP 58000, Morelia, Mich, México, Tel +52-4433223500
Received: June 28, 2024 | Published: July 15, 2024
Citation: Saucedo-Martínez C, León-Balderas I, Cruz JLI, et al. Genetic characterization of bacteria in bioremediation of soil polluted by waste motor oil: safe vegetal consume. J Appl Biotechnol Bioeng. 2024;11(4):89-92. DOI: 10.15406/jabb.2024.11.00365
Waste motor oil (WMO) is a mixture of insoluble aliphatic hydrocarbon molecules with chains between C11-C20 and C20-C35, as well as of aromatic, polycyclic and halogenated compounds. In Mexico, this mixture is classified as hazardous waste, according to the “General Law of Ecological Balance and Environmental Protection". WMO is generated in mechanical workshops due to use in agricultural machinery and its inadequate final disposal that occasion an environmental problem. WMO in soil has negative effects that limit agricultural production including health problem in humans. An alternative solution is biostimulation, first with biodetergents that emulsified them, followed of enrichment with a mineral solution that contents nitrogen, phosphorus, and potassium. The effective mineralization of WMO is based in the fact that soil is a source of diversity of bacteria able to eliminate WMO. The aims of this research were: i) to isolate and select bacteria capable oxidate WMO due that biodetergent and lipolytic activity and ii) the genetic identification of these soil bacteria involved in oxidation WMO. Results show that some microorganisms were isolated from soil contaminated by two concentrations of WMO, from soil contaminated by 60,000 WMO: Achromobacter denitrificans, a short Gram-negative rod, two species of the sporulated genus Gram-positive Bacillus, B. horneckiae and B. subtilis, and an actinomycete Gordonia amicalis, a Gram-positive coccobacillus. From soil contaminated by 80,000 ppm and WMO, another member of the Bacillus genus, B. cereus strain 2, was isolated and according to the molecular identification that places them as part of a metagenome it has the capacity to synthesize detergents and with ist lipolytic activity to oxidant WMO. These results support that in agricultural soils contaminated by waste motor oil there is a diversity of bacteria, which synthesize biodetergents simultaneously with lipolytic activity, both useful tools for soil bioremediation and the recovery of healthy plant productive capacity, safe for human and animal consumption.
Keywords: mix hydrocarbons, soil, pollution, bacteria, genetic diversity, bioremediation, safe agriculture production
WMO, waste motor oil, LDC, lysine decarboxylase; ODC, ornithine decarboxylase; p-NP, p-nitrophenol
The contamination of agricultural land by hydrocarbons such as waste motor oil (WMO) in Mexico reaches up to 55%,1 which occasion an environmental problem in Mexico and the world. The toxicity of WMO decreases agricultural production and the General Law of Ecological Balance and Environmental Protection2 considers it as a dangerous waste, while the Mexican standard NOM-138-SEMARNAT/SSA1-20123 establishes a maximum limit of 4,400 ppm in agricultural soil. When WMO pollutes the soil, it inhibits microbial life with loss of plant fertility.1,4,5 WMO contains insoluble aliphatic, aromatic and polycyclic hydrocarbons, that do not easy to mineralize, as consequence there is risk for humans and animals.5,6 The ecological solution is the biostimulation with synthetic and microbial detergents that it them emulsify and after to oxidizing at the WMO by its lipolytic activity.7,8 In soil polluted by WMO or any hydrocarbons mix, it is possible to isolate aerobic heterotrophic microorganisms that could induced the oxidation of WMO with a mineral solution according to the chemical properties of the soil.9,10 By doing so any agriculture soil, could be biorecovery by biostimulation which included microorganisms and plants (phytoremediation).11 Since it has been showed that there is, wide diversity of bacteria able to oxidate at the WMO,12,13 the objectives of this work were: i) isolate and select bacteria capable oxidant WMO by its biodetergent and lipolytic activity and ii) genetic identification of the soil bacteria involved in oxidation WMO.
Isolation of microorganisms from agricultural soil contaminated by waste motor oil (WMO)
All microorganisms’ isolates were recovered from agricultural soil polluted by 60, 000 and 80,000 ppm of WMO, each 10 g soil samples were diluted in 100 ml saline solution at 0.85 %, shacked for 15 min and then were cross streaked in WMO agar media. The medium WMO agar had following composition (g/L): casein peptone 5, yeast extract 1, KH2PO4 2.5, K2HPO4 2.5, MgSO4 2.5, NaCl 1.0, trace element solution 1 mL, waste motor oil 10 mL, 0.5% commercial detergent. The pH was adjusted to 7.0 and agar was added at 18.0 g/L. The cultures were incubated at 30oC/48 h. When axenic cultures in WMO agar medium grew were taken different colonies and each microorganism was store in sterile soil. These microorganisms were identifying based on its biochemical and genetic profiles and its ability for releasing biodetergents and lipolytic activity related to its ability to oxidant WMO.
Biochemical identification of bacterial isolates from soils contaminated by 60,000 and 80,000 ppm of WMO. Each microorganism was observed after 30 h, to be sure that it was pure and viable for subsequent biochemical identification. Based on the examination of the microscopic morphology and Gram stain, it revealed different types of microscopic morphology, short and long rods, as well as sporulates. In WMO agar, the axenic colonies were suspended in 0.85% saline solution to determine its profile biochemical with the following tests: oxidase, catalase, H2S production, lipase, lysine decarboxylase (LDC), citrate, urease and ornithine decarboxylase (ODC) using the compact Vitek system.14
Synthesis of biodetergents and activity lipolytic in genera and species isolated from soil contaminated by WMO
These various genera and species of microorganisms most common or dominant in both agricultural soils contaminated by waste motor oil were subjected to the double test of biodetergent synthesis and lipolytic activity that is necessary for them to be able to eliminate WMO. The WMO was obtained from a mechanical workshop in the city of Morelia, Michoacán, Mexico, while the lubricating oil was Mobil Super 15w40 Multigrade Trc-pro 946 MI. A mineral medium was prepared with WMO and lubricating oil at 1% v/v, as the only source of carbon and energy for the induction of biodetergent synthesis and lipolytic activity for the WMO mineralization. All isolates were activated on the following chemical composition (g/L): WMO or lubricating oil 10, 0.5 of NH4NO3 or NaNO3; 1 of K2HPO4, KH2PO4, MgSO4, NaCl, FeSO4 and CuSO4.0.5. Bacterial cultures were incubated at 30 °C for 48 h at 250 rpm, then 1 ml aliquots were taken and filtered on 0.22 µm membranes, to obtain the cell-free supernatant where the enzymes were found. Extracellular lipases were quantified spectrophotometrically, using p-nitrophenyl palmitate (p-NPP) (Sigma-Aldrich) as substrate according to7. In this method, the hydrolysis of p-NPP to release p-nitrophenol (p-NP) was measured. Thirty mg of p-NPP was dissolved in 10 ml of isopropanol and 90 ml of 100 mM Tris-HCl at pH 8.0. From this mixture 150 μL was taken and the reaction was initiated by adding 100 μL of the cell-free filtrate grown in mineral medium with WMO and lubricating oil at 1% v/v. After 1 h incubation at 37°C, absorbance was measured at 405 nm against a control containing the same components except the cell-free filtrate. One unit of enzyme activity was defined as the amount of enzyme releasing 1 μmol of p-nitrophenol per minute under the assay conditions12. While anionic biodetergents were measured by the standard method for methylene blue for determination of anionic surfactants modified and combined with spectrophotometric measurement. The developed method was found to be satisfactory in terms of sensitivity and precision, with a short time of analysis. The quantification limit for anionic surfactants was at 16 μg L−1, with a relative standard deviation of 1.3 % for a model sample and 3.8 % for any sample7. All experimental data were analyzed by ANOVA-Tukey HDS at 0.05%15
Molecular identification of biodetergent-generating and lipolytic activity in bacteria isolated from soil contaminated by WMO
At this stage of each axenic culture of WMO-oxidizing bacteria that synthesized detergent, the extraction and purification of genomic DNA was performed with Monarch Genomic DNA Purification Kit, New England Biolabs. The DNA was analyzed by electrophoresis with 1% agarose gel, the V3 hypervariable region of the 16S rRNA gene was amplified with 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-TACGGYTACCTTGTTACGACTT-3′ oligonucleotides. The reaction conditions for the thermocycler were denaturation of DNA at 95 ºC/1 min, followed by 10 cycles at 95 ºC/30 s, annealing 65-60 ºC/30 s and an extension step at 72°C/1 min. To separate and visualize the 16S rRNA amplicons, electrophoresis was performed with a 1% agarose gel, which was run at 80 V/30 min. At the end, the gel was observed in a UV transilluminator, and the molecular identification was carried out in Molecular Bacteriology Laboratory, CINVESTAV Unidad Irapuato, Gto., México. The sequences obtained were analyzed, taxonomically classified with a distributed BLASTn.NET algorithm12 against a database of high quality 16S rRNA sequences obtained from NCBI.7 The BIOLOG GENIII Identification System was used for B. cereus strain 2 isolated from soil contaminated by 80,000 ppm of WMO. A bacterial suspension of B- cereus strains 2 at 85% transmittance was prepared using the IFA inoculation fluid, and the plate was incubated at 28°C for 24 h. The tests were carried out in triplicate. Each of the plates was evaluated and the color reaction indicating the use of the substrates for each of the strains was registered. The results were subsequently captured in the database.7,12
Main bacteria isolated from agricultural soil contaminated by WMO
Three Gram-positive rods were isolated, two sporulated and another non-sporulated, a Gram-positive actinomycete rod, and a Gram-negative rod, the only one from agricultural soil contaminated by 60,000 ppm of WMO. From soil contaminated by 80,000 ppm of WMO, the dominant sporulated Gram-positive rod was isolated. These results are interesting because usually are Gram-negative rods that are isolated in environments contaminated by hydrocarbon mixtures as well as WMO, that have undergone bioremediation.8,12 Research in progress is trying to understand why.
Biochemical profile of bacteria isolated from agricultural soils contaminated by WMO
The result of the identification by biochemical profile of the bacterial isolates from soil contaminated by 60,000 ppm of WMO showed the existence of A. denitrificans, a short Gram-negative bacillus, of B. horneckiae, a Gram-positive rod with subterminal spores, and the other species of sporulated Bacillus was subtilis was also Gram-positive, both species native to the soil. In contrast to the actinomycete G. amicalis another native Gram-positive coccobacillus. Meanwhile, from the soil contaminated by 80,000 ppm of WMO, the biochemical was isolated and identified as the dominant genus and species, B. cereus strain 2, a long Gram-positive sporulated rod, also native to the soil.13
Table 1 shows the test carried out with the genera and species of bacteria isolated from soils contaminated by 60,000 and 80,000 ppm of WMO, which to mineralize it, synthesize biodetergents and lipases induced by the lubricating oil and the WMO. All five genera and species of bacteria synthesized biodetergents of anionic nature surfactin type. B. cereus strain 2, registered 0.41ml and A. denitrificans 0.50 ml of anionic biodetergents/100 ml of medium. Surfactin is an anionic cyclic lipopeptide composed of an amino acid chain linked to a fatty acid chain,12 which has been reported as one of the most common biodetergents synthesized by this genus and species of bacteria. In the case of lipolytic activity A. denitrificans reached an activity of 279 U/mL induced by WMO and 200 U/mL in mineral medium induced by lubricating oil. G. amicalis registered a lipolytic activity of 199.9 U/mL induced by WMO, and 182.3 U/mL with lubricating oil, numerical values statistically different from the uninoculated mineral medium where no lipolytic activity was registered. The results show that these genera and species of bacteria synthesized a wide diversity of biodetergents and presented a specific lipolytic activity for the emulsification and the hydrolysis of a diversity of WMO hydrocarbons aliphatic and aromatic from 15 to 50 carbons and 34 to 90 times more aromatic hydrocarbons than the lubricating oil,8 which registered an average hydrocarbon composition of 7 to 26 aliphatic and aromatic carbons9. These results confirm that A. denitrificans, B. horneckiae, B. cereus, B. subtilis, G. amicalis, could synthesize biodetergents and lipases induced by WMO for adequate temperature and oxygenation level. The results showed that these genera and species have potential to synthesis crude extracts of biodetergents and lipases, which to emulsifing and hydrolyzing them.10 Due that extract crude of biodetergents and lipases as part of the strategies for soil bioremediation polluted by WMO, now we have a promising action to solve this type of problem of environmental pollution caused by mixtures of hydrocarbons as well as WMO.8
|
Genus and species of bacteria identified by genetic characterization |
Mineral medium plus lubricating oil |
Mineral medium plus waste motor oil |
||
|
Concentration of anionic biodetergnt (mL/100 ml of medium) |
Lipolytic activity (U/ml) |
Concentration of anionic biodetergent (mL/100 ml of medium) |
Lipolytic activity (U/ml) |
|
|
Control |
0e* |
0c |
0e |
0c |
|
+Achromobacter denitrificans |
0.50b |
200.0b |
0.58b* |
279.09ª |
|
+Bacillus horneckiae |
0.33d |
121.1f |
0.39d |
188.1e |
|
++Bacillus cereus strain 2 |
0.41c |
199.7a |
1.06a |
249.8b |
|
+Bacillus subtilis |
0.38d |
160.5d |
0.38c |
239.0b. |
|
+Gordonia amicalis |
1.05ª |
182.3c |
1.04a |
199.9d |
Table 1 Ability to synthesize biodetergents and lipolytic activity of genera and species of bacteria isolated from soil contaminated by waste motor oil and lubricating oil
*Different letters are statistically different according to ANOVA-Tukey HSD at 0.05 %.
+= isolates from soil impacted by 60,000 ppm and ++ isolated form soil impacted by 80,000 ppm of WMO
Molecular identification of bacteria isolated from soil contaminated by WMO
Through the metagenomic analysis of soil contaminated by 60,000 ppm of WMO, this phylogenetic tree was created, based on ribosomal RNA (Figure 1). It is possible to see A. denitrificans, B. horneckiae, B, subtilis and G. amicalis are distributed in strategic parts of this tree which demonstrates that the capacity for biodetergent synthesis and lipolytic activity is shared by a large group of genera and species that have soil as one of their main habitats. By doing this genetic analysis a proteobacteria was detected, considered new oxidant species of WMO, through the synthesis of biodetergents and lipolytic activity as A. denitrificans, that belongs to the group of proteobacteria already recognized for synthesizing lipid-polysaccharide biodetergents.7,10 The genetic relationship between these genera and species of bacteria isolated from soil contaminated by 60,000 of WMO, that requires all them to synthesize biodetergent and lipolytic activity in these bacterial isolates, has a potential value in the augmentation of soil contaminated by hydrocarbon mixtures such as WMO, for recover an agricultural soil where plant production is not negatively affected nor is there a risk to the health of humans and animals when consuming these vegetables grown in a soil that was previously bioremediated.13
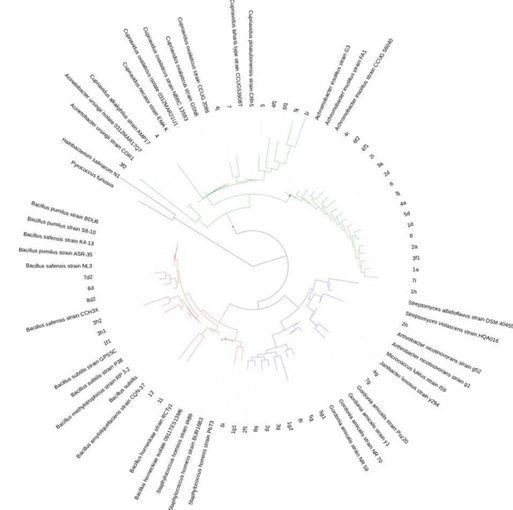
Figure 1 The phylogenetic tree was created through of metagenomic analysis of soil contaminated by 60,000 ppm of WMO, based on analysis of V3 hypervariable region of 16S rRNA. It is possible to see that A. denitrificans, B. horneckiae, B, subtilis and G. amicalis are distributed in strategic parts of this tree.
B. cereus strain 2 isolated from soil contaminated by 80,000 ppm of WMO, was located in a different branch associated with other types of species of the Bacillus genus such as B. thuringiensis that has the ability to synthesize biodetergents and lipid activity (Figure 2). These suggests that in nature, the hydrocarbon mixtures as well as WMO, could be used as carbon energy source and makes this genus be necessary by its capacity to colonize soil contaminated by WMO.7
Based on the evidence, the wide distribution of genera and species of actinomycetes and bacteria that are part of the native population of agricultural soil that has been contaminated, will allow the elimination of WMO to a level that not only complies with NOM-138 through bioremediation by biostimulation, but it guarantees that agricultural production be safe for human and animal consumption, while the WMO remaining in the soil, especially the aromatic toxic fraction, disappears.
Experimentation BCSN, IBL and JLIC; conceptualization JMSY, EMBP; EMBP methodology, JMSY and software, JMSY and JLIC validation, JMSY; results BCSM, IBL, JLIC, and JMSY; investigation, EMBP and JMSY; resources, EMBP, JMSY a writing original draft preparation JMSY and EMBP writing—review and editing, JMSY and EMBP visualization JMSY; supervision JMSY. All authors have read and agreed to the published version of the manuscript.
Thanks to project 2.7 (2024) supported by the Scientific Research Coordination (CIC)-UMSNH, to Phytonutrients de México, to BIONUTRA S.A. de C.V., Maravatio, Mich, México and to project CBF-2023-2024-2234 supported by Conahcyt.
The authors declare no conflicts of interest.
None.

©2024 Saucedo-Martínez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.