Journal of
eISSN: 2572-8466


Research Article Volume 6 Issue 4
1Department of Plant Science, Autonomous University Chapingo, Mexico
2Thesis Master of Science in Biotechnology, Department of Plant Science, Mexico
3Professors Autonomous University Chapingo, Mexico
Correspondence: Rodriguez de la O JL, Academy of genetics, Department of Plant Science, Autonomous University Chapingo, MexicoCarr. Fed. Mexico Texcoco Km 38.5 CP 56230, Mexico
Received: July 10, 2019 | Published: August 12, 2019
Citation: la RDOJL, Flores LJVA, Monterrubio RMS, et al. Evaluation of responses embryogenic Cycas revoluta thumb., from callus culture obtained in vitro. J Appl Biotechnol Bioeng. 2019;6(4):188-192. DOI: 10.15406/JABB.2019.06.00192
Processes cell differentiation and dedifferentiation are included in the development of biotechnology protocols to promote somatic embryogenesis as an alternative to the in vitro propagation of plants, somatic embryos may be an excellent strategy for both propagation and conservation of fossil species such as cycads. They were evaluated in vitro with different strategies, morphogenic responses associated with obtaining somatic embryos of C. revolute. calli of megagametophytes, subsequently subcultured in four combinations of basic salts of Murashige and Skoog (1962) MS, with the addition of benzyladenine (BA), and 2, dichlorophenoxyacetic (2,4-D) were used, and kinetin (K), and picloram. In the results, It was possible to characterize potentially embryogenic callus, evaluating the levels of both cellular differentiation, necrosis, texture and color; and increases mass or weight considered to start differentiation or proembryogenic or globular type. Calli were subcultured in a medium containing MS salts, incorporating abscisic acid (ABA) in 0, 0.38, 1.13, 3.78 and 5.67 uM doses influenced both the production and maturation of somatic embryos. Embryonic structures, presented a pink coloration characteristic strongly associated towards maturity. The effect of combinations of BA, Kin, 2,4-D, GA3 and ANA influenced the development and germination of mature somatic embryos. And the combination of 1.36 mM 2,4-D+4.44 uM BA promoted the appearance of calluses with a compact texture, characteristic related to their embryogenic potential. The purpose of this research in Cycas sp was to contribute to the study of the in vitro morphogenic responses of this group of plants. And somatic embryogenesis, will allow the obtaining and multiplication as well as its preservation of Cycas sp. Gender that is evolutionary very important.
Keywords: revoluta, cycad, somatic embryogenesis, growth regulators
Cycads are a group of ancestral dioecious, which appeared in the pérmico and evolved from the progymnosperms free spores, preceding the Gingkoales and Gnetales, succeeded in developing mechanisms of adaptation and survival, so have been called "living fossils". Biologically are very interesting because they represent an important stage in the evolution of flowering plants and are considered baseline in the evolutionary tree seed plants.1 Their beauty and rarity, has generated a great demand, so we have become perfect target of ends and people dedicated to the black market collectors of exotic species, causing today all species of the order Cycadales are included in Appendix I or II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES, 2017). C. revolute It is the oldest living cycads and is included in Appendix II of CITES (2017). The species symbolizing economic importance of order,2 ornamental and besides, they have been attributed to nutritional and medicinal properties with clear antibacterial and deleterious effects against colon cancer and epidermoid.3–5 However, due to slow growth, dioicismo, development, and contradictions in the seed, the spread is directed to the use of buds or buds in the basal part of the parent plants limiting genetic variability significantly. Thus, It has emerged a clear need and unpostponable the use of biotechnological tools that enable effective propagation of the species to meet demand, and contribute to the conservation and avoid extinction. The ES is the in vitro development of embryos from cell culture are not the product of a gametic fusion.6 In the last 70 years are few reports in vitro cultivation in these plants. Somatic embryogenesis in cycads is relatively low and the best responses were obtained from ZE.7 The morphogenic potential tissue in cycads has been recognized for many years and that the use of haploid explants or those that do not require fertilization, can significantly optimize genetic resources, since there are species such as Encephalartos woodii Sander., of which only exemplary and have male sexual propagation is impossible. C. revolute investigations have sought regeneration from ZE;8,9 leaf midrib and epicotyls;10 endosperm11 and megagametophytes; however, the use of megagametophytes as a source of explant organogenics responses generated (buds and roots), according Ashmore12 are limited for conservation of genetic resources use due to genetic instability inherent to these plants. EPE development from embryogenic cultures in cycads, It remains inefficient since cultures cannot be synchronized and therefore certain developmental events are difficult to identify. Necrosing problems are frequent and slow responses due to the nature of this type of plant; Litz et al.,7 have indicated that several species of embryogenic cultures have grown cycad vigorously and are highly morphogenic after 11 years of induction. Growth regulators can promote morphogenesis even if the salt concentration is not adequate. However, when it is desired to develop ES, use of regulators depends on the stage of the process (induction, proliferation, maturation or germination). While in cycad it has suggested the use of 2,4-D induction, Embryogenic cultures should be subcultured in the same formulation of basal medium but lacking growth regulators. Litz et al.,7 they founded that these embryogenic cultures are not receptive to ABA because of its evolutionary condition in the plant kingdom. However, it is essential to remember that many factors other than growth regulators, influencing embryogenic responses, such as the species and type of explant. Therefore, the objective of this study was to establish the conditions in vitro that can promote the main morphogenic responses associated with obtaining somatic embryos of Cycas revolute Thunb., Which allow to establish a protocol alternative propagation and preservation and contribute to study of these species.
The research was conducted at the Laboratory of Plant Tissue Culture Department of Plant Science at the Autonomous University Chapingo, Mexico. calli three months old were used obtained from in vitro culture of C. revolute megagametophytes from a culture medium with macroelements and salts of B5 medium13 and microcells MS medium (1962) supplemented with arginine, asparagine and glutamine (100, 100 and 400mg L-1, respectively), 6% sucrose and 6 g L-1 gellam-gum. Calli were obtained under the effect of two combinations of auxin-cytokine (Table 1). The process for promoting somatic embryogenesis was evaluated in several experimental stages and each strategies., Considered as the induction, maturation and germination of embryos obtained.
Treatment |
Growth regulators (uM) |
|
|
||
2,4-D |
picloram |
Kin |
BA |
TDZ |
|
one |
9.05 |
- |
13.93 |
- |
- |
two |
- |
27.75 |
- |
2.13 |
2.22 |
Table 1 Types and auxin-cytokine concentrations applied to megagametophytes of Cycas revolute for callus induction
Promotion stage callus. Mass increases, cell differentiation, and obtaining globular structures or proembriogenics. Embryogenic calli were subcultured to promote both their differentiation and embryonic responses using different strategies based, the medium with the inorganic salts of Murashige & Skoog14 MS (1962), by changing the presence or absence of auxiliaries and regulators increase.
Strategies |
Treatment |
Growth regulators employees (uM) |
|||
BA |
2,4-D |
Kin |
picloram |
||
one |
0 |
- |
- |
- |
- |
one |
1.33 |
0.45 |
- |
- |
|
two |
4.44 |
1.36 |
- |
- |
|
3 |
13.32 |
4.52 |
- |
- |
|
two |
0 |
- |
- |
- |
- |
one |
- |
0.45 |
2.32 |
- |
|
two |
- |
2.26 |
4.65 |
- |
|
3 |
- |
6.79 |
13.94 |
- |
|
3 and 4 |
0 |
- |
- |
- |
- |
one |
- |
- |
0.46 |
4.14 |
|
two |
- |
- |
2.32 |
4.14 |
|
3 |
4.65 |
4.14 |
|||
Table 2 Types and concentrations of growth regulators applied to amorphous callus Cycas revolute in proliferation step
Variables evaluated
To identify the physical characteristics of callus associated with their embryogenic potential was established a model to determine levels of cellular organization or differentiation, until the formation of globular structures or proembriogenics type, having as variables.
Increase in mass (weight) of the callus (IB)
Both the number of embryonic structures and levels of cell differentiation DC, and the levels of blackening or oxidation and color callus were evaluated every ten days using a stereomicroscope Carl Zeiss Stemi DV4 with a lens (8x/21) for 80dds. IB biomass increases in corns, was taking his weight using an analytical balance every month for three months. In each experimental distribution strategy remain four treatments a Table 2 and with 10 repetitions per treatment. Each experimental unit was a Gerber type flask with 20 ml of culture medium.
Obtaining step and embryo maturation
Callus cell differentiation levels DC were transferred to 3/2 MS medium (1962) by diluting 75 and 25% of nitrate source (N03) and ammonium (NH4), respectively. The medium with a pH of 5.7±0.1 was added glycerol (30 g L-1), glutamine, arginine, asparagine, L-cysteine, PVP (400, 100, 100, 60 and 70mg L-1) and sucrose one%. Incorporating Ac. Abscisic (ABA), in concentrations of 0, 0.38, 1.13, 3.78 and 5.67μM. The experiment was set under a completely randomized design with 10 replications per treatment. Each experimental unit was a Gerber type flask with 20 ml of culture medium.
Variables evaluated
As variable was the formation of mature somatic embryos obtained every ten days, counted with a stereomicroscope Stemi DV4 Carl Zeiss. Calluses They were incubated with 16 hour photoperiod light (26°C) and 8 hours darkness (24°C).
Stage, germination and development of somatic embryos
Mature somatic embryos were subcultured on MS basal medium (1962) with a pH of 5.7±0.1, supplemented with myo-inositol, thiamine, pyridoxine, folic acid, biotin, Polivinylpilorridone (PVP), mannitol (100, 0.40, 0.50, 0.50, 0.50mg L-1), 4% sucrose and 7mg L-1 agar. Evaluating the percentage of embryos with responses associated with maturity and germination, evaluating the set of auxins, cytokinins and gibberellins (Table 3) effect. Calli were incubated under the same conditions of light and temperature in step above.
Treatment |
Growth regulators (μM) |
|
|
||
2,4-D |
BA |
Kin |
ANA |
AG3 |
|
0 |
- |
- |
- |
- |
- |
one |
- |
- |
4.65 |
1.61 |
1.44 |
two |
- |
- |
13.93 |
5.37 |
2.88 |
3 |
1.33 |
0.45 |
- |
- |
- |
4 |
4.44 |
1.35 |
- |
- |
- |
5 |
13.32 |
4.53 |
- |
- |
- |
Table 3 Types and concentrations of growth regulators used in the germination of somatic embryos of Cycas revolute
Statistic analysis
To determine the effect of growth regulators in vitro responses embryogenic callus cultivated on an analysis of variance for variables CE and IB, the proliferation phase is performed; and number of mature embryos with a completely randomized design with Tukey test with α=0.05 and comparison of means using SAS (Statistical Analysis System, 2002).
The statistical model used was:
where: Yij, Variable Response; μ, average general; Tij, Effect of growth regulators; ij, experimental error.
For variables: DC levels, blackening, necrosing (oxidation), color and consistency, the proliferation stage, pictures with the Chi-squared Pearson (X2) were performed.
The calli were subcultured in basic medium with the inorganic salts of MS (1962), for each of the steps aimed at obtaining somatic embryos, and according to the results obtained, the concentration of salts and mainly the type and concentration growth regulators employed influenced decisively on this particularly that regulators can promote embryogenic responses, even when the concentration of salts is not adequate.
Strategy 1: Nitrate reduction 50% MS medium (1962), did not influence the formation of structures proembrionarias EPE, before 80 dap; however, the combination of regulators BA+2,4-D promoted enhanced differentiation of embryonic structures. Noting that the top level of cytokinin BA, limits the growth of calluses in their increasing mass, but promotes cell differentiation processes, to obtain proembrionarias structures (IB), as shown by the results with α=0.05 , that there were significant differences in the number of EPE arising under different doses employed BA+2,4-D (Table 4); However, the variability obtained is due to the effect of treatments, as described Fieire (2003), which explains that embryonic responses are grouped characteristics callus cultured.he combination of 4.44 uM +1.36 (BA+2,4-D), had a higher response in obtaining embryonic structures (average 2.30), at 80dds. (Figure 1). Thus, the behavior was callus EPE concerning the number of the most obvious development period.
Concentration BA + 2,4-D (M) |
Biomass increase (mg) |
Number of different structures |
0 + 0 |
88.44 to▪ |
0.00 b▪ |
1.33 + 0.45 |
86.82 to |
0.00 b |
4.44 + 1.36 |
72.26 to |
2.30 to |
13.32 + 4.52 |
61.79 to |
0.8 ab |
Table 4 Effect of four levels BA +2,4-D on the amount of callus and number of structures proembriogenics Cycas revolute to 80 dap
▪Valores with the same letter within columns are equal according to the Tukey test at P≤0.05.
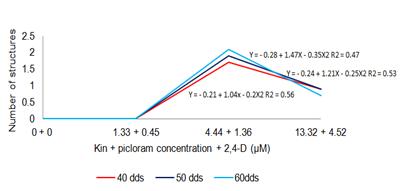
Figure 1 Schematic comparison of the development of EPE (40-60 dds) of Cycas revolute, under the effect of BA +2,4-D.
Strategy 2: The basic MS medium (1962) over the combination of kinetin+2,4-D promoted more EPE compared to other strategies co callus culture in the differentiation stage. Statistically significant differences (α=0.05) IB when the concentrations of kin+2,4-D increased; however, compared to the results obtained in strategy 1, treatment stimulated greater IB, also generated a higher number of EPE (Table 5); recognizing the effect of promoting embryogenesis due to the presence of 2,4-D coinciding with Cangahuala et al.,15 and Konieczny et al.,16 that evaluated this effect, regarded as one of the factors that will determine the ES promotion rates. Comparison of means showed greater variability treatment, indicating precisely one ESAF. The number of EPE was a significant increase according to the concentration of growth regulators used, evident from dds 40 (Figure 2).
Concentration KIN + 2,4-D (μM) |
Biomass increase (mg) |
Number of different structures |
0 + 0 |
44.00 b▪ |
0.00 to▪ |
2.32 + 0.45 |
73.74 b |
2.10 |
4.65 + 2.26 |
ab 143.53 |
2.70 to |
13.94 + 6.79 |
236.96 to |
10.00 |
Table 5 Effect of four levels kin + 2,4-D on the amount of callus and number of structures proembrionarias Cycas revolute to 80 dap
▪Values with the same letter in column are equal according to the Tukey test at P≤0.05.

Figure 2 Schematic comparison of the development of EPE (40-60 dds) of Cycas revolute, under the effect of kin+2,4-D.
Strategy 3: MS (1962) medium diluted 50%, the average development allowed EPE 1.30 to 80 dds in the absence of growth regulators. Meanwhile combining 2.32+4.14 uM (kin+picloram) stimulated average 5.5 differentiated structures (of which 25.45% were poorly differentiated structures development. The increased cell differentiation under the effect of 0.46+4.14 mM (kin+picloram), was reflected in a numerically greater IB, the high concentration auxin versus cytokinin source (Table 6). Production structures differentiated under the effect of 2.32+4.14 uM (+picloram kin) was higher after 40 dds (Figure 3).
Concentration of kin + picloram (uM) |
Biomass increase (mg) |
Number of different structures |
0 + 0 |
109.9 a▪ |
1.30 to▪ |
0.46 + 4.14 |
580.9 to |
1.70 to |
2.32 + 4.14 |
390.8 to |
5.50 to |
4.65 + 4.14 |
321.6 to |
3.50 to |
Table 6 Effect of four levels kin + picloram (50% salts) on the amount of callus and average number of proembrionarias structures Cycas revolute to 80 dap
▪Valores with the same letter within columns are igualdes according to the Tukey test with a P≤0.05.

Figure 3 Schematic comparison of the development of EPE (40-60 dds) of Cycas revolute, under the effect of kin+picloram (inorganic salts 50%).
Strategy 4: The basic MS medium (1962) diluting nitrate 50% without the presence of growth regulators, allowed development of average 1.10 EPE. Moreover employed combinations of kin+picloram developed statistically significant responses (α=0.05) in IB and number of EPE dds 80 (Table 7). Figure 4 shows the production of EPE upward under the effect of the doses used kin+picloram from 40 dds.
Concentration of kin + picloram (uM) |
Biomass increase (mg) |
Proembrionarias number of structures |
0 + 0 |
136.2 b▪ |
1.10 b |
0.46 + 4.14 |
803.8 ab |
5.50 to |
2.32 + 4.14 |
925.6 to |
ab 2.80 |
4.65 + 4.14 |
659.3 ab |
1.90 b |
Table 7 Effect of kin + picloram levels (source of nitrate 50%) on the amount and number of callus proembrionarias structures Cycas revolute to 80 dap
▪Values with the same letter in column are equal according to the Tukey test with a P≤0.05.

Figure 4 Schematic comparison of the development of EPE (40-60) dds Cycas revoluta, under the effect of kin+picloram (source of nitrate 50%).
Physical characteristics of calluses
The combination of BA+2,4-D in the MS (1962) with the source of nitrate diluted to 50%, stimulated the development of callus with a level of DC (2) having 50%; nodular characteristics and little response to the formation EPE. However, statistically significant differences with α=0.05 between the necrosing and the texture type and coloring according to the concentrations of growth regulators used, including the control (Table 8) were presented. Accepting cytokinins high doses promotes accumulation of phenolic compounds by Abohatem. So 13.32μM BA presented 3 necrosing levels. The effect of kin+2,4-D in the MS medium (1962), promoted the development of calluses a DC level 2; However, unlike strategy 1, it expressed a clear friable texture in 72.5% of the calli and necrosing of 47.5%. I exist significant differences with α=0.05 in the color corns between treatments, including the control (Table 8). MS basal medium (1962) with reducing salts 50%, and the effect of kin+picloram, calli promoted structures with differing levels of 45% and compact texture at 70%; however, statistically significant differences, with α=0.05 in the necrosing between treatments, including the control (Table 8). Incorporating joint kin+picloram to MS medium (1962), 50% reducing nitrate source, compact calli produced by 72.5%. Statistically significantly different at α=0.05 level of DC (Figure 5).
|
statisticians |
variables |
|||
MS mode (1962) |
Levels of cell differentiation |
Necrosing levels |
Coloration |
Consistency |
|
one |
P-value |
0.13 |
0.01* |
0.00* |
0.01* |
|
X2 |
2.51 |
2.26 |
0.02 |
0.09 |
two |
P-value |
0.71 |
0.17 |
0.01* |
1.00 |
|
X2 |
10.80 |
2.86 |
0.84 |
- |
3 |
P-value |
0.18 |
0.03* |
0.20 |
0.95 |
|
X2 |
2.90 |
1.25 |
5.38 |
8.00 |
4 |
P-value |
0.03* |
0.04* |
0.01* |
0.48 |
|
X2 |
1.27 |
3.13 |
4.81 |
2.27 |
Table 8 Significance obtained by Pearson X2 test in the evaluation of statistically significant contrast between treatments mode in proliferative stage callus Cycas revolute to 80 dap
*, P≤0.05 statistically significant differences between treatments.
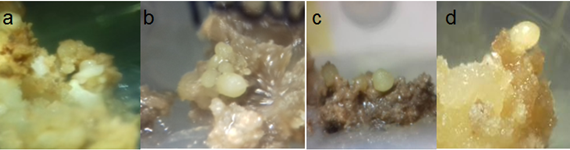
Figure 5 Development of Cycas revolute proembrionarias structures 70 days old at the MS (1962) proliferation step. A) 4.44+1.36 uM (BA+2,4-D; nitrate 50%); B) 13.94+6.79 uM (kin+2,4-D, salts 100%); C) 2.32+4.14 uM (kin+picloram, salts 50%); D) 2.32+4.14 uM (kin+picloram; nitrate 50%).
Obtaining somatic embryo maturation
The combination 4.44μM 1.36μM BA+2,4-D and diluting MS inorganic salts (1962) 50%, potentially promoted obtaining embryogenic callus, to 170 dds, with differing levels of DC (2), color (2) and compact texture (Figure 6), initially calluses were cultured on MS medium (1962), without the presence of growth regulators, and light. The presence of regulators in the doses mentioned, promoted embryonic structures from 10 dds and pink. Differentiation was progressive from 40 days (Table 9); and maturity from 80 dds. According to their histology was observed on meristematic activity of cells in apical zones. 6, the development of pre-cotyledon and cotyledon structures presented in some somatic embryos (Figure 8). Embryogenic developments C. hildae obtained from the culture of leaves of young leaves.EPE 80, 60, and 100 dds, generated in the means of proliferation stage were subcultured to maturity; however, responses were not satisfactory due to the presence of auxin in some treatments inhibiting subsequent embryonic development as reported Vinas and Jimenez.16 Also, tripe with DC (2) level less than 100 days old, were subcultured in the same medium and showed no embryonic responses, indicating that the time of the callus culture is essential in obtaining somatic embryos, as reported by Ruiz.18

Figure 6 A) Embryogenic calli of Cycas revolute obtained by combining 4.44 uM BA+1.36 .mu.M 2,4-D; B) Polyembryony observed in medium without growth regulators.
ABA dose (μM) |
Days after subculture (dds) |
|||
10 |
twenty |
30 |
40 |
|
0 |
1.70 |
2.60 |
3.00 |
3.00 |
0.38 |
1.00 |
1.30 |
1.90 |
2.20 |
1.13 |
1.00 |
1.20 |
1.70 |
1.90 |
3.78 |
0.60 |
0.90 |
1.20 |
1.40 |
5.67 |
0.60 |
0.80 |
0.90 |
0.90 |
Table 9 Maturation of somatic embryos of Cycas revolute expressed in the maturation medium under five concentrations of ABA

Figure 7 Embryogenic callus responses in Cycas revolute in medium without growth regulators; A) development of rosacea coloration and pro-embryos (10 dds); B) globular embryonic structures immature (30 DAP); C) Callus (70 dds); D) differentiation of somatic embryos with (70 dds) suspensor evident; E) the presence of pre-cotyledon structures (70 dds); F) cotyledon (110 dds).
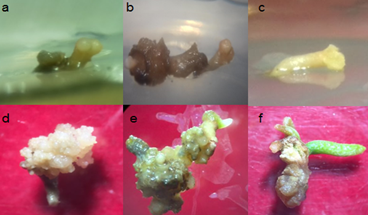
Figure 8 Production of somatic embryos of Cycas revolute with the effect of 4.44+1.35 uM (BA+2,4-D). ab) bipolar meristematic growth evident from the 10 (A) and 40 dds (B); apical meristem growth and radical 10 (C) and 220 dds (D); E) coleoptilar expression of an embryo of 27 dap; F) development of primary leaflets, apparent photosynthetic activity at 70 dds (F).
The use of ABA in culture medium showed that the higher the dose, the lower the number of developed somatic embryos (Table 9); meanwhile, von Arnold et al.,19 mentioned that ABA can promote the synthesis of storage reserves in embryos during maturation; however, Litz et al.,7 reported that somatic embryos in cycads are not receptive to ABA because of its evolutionary condition in the plant kingdom. The observation of the gradual development of somatic embryos obtained from C. revolute, allowed to show their morphological development is similar to that expresses a ZE, since the development of pro-embryos (being those which showed internal progress) erythrocyte and Torpedo type until the pre and cotyledon stage (Figure 7).
Germination of somatic embryos
Embryonic structures 129 days old, had responses associated with the development and germination by 90% under the combined effect of 4.44+1.35 uM (BA+2,4-D). Germination is a naturally slow process; and also under in vitro conditions; in Figure 8, embryonal observed at 10, 40 and 220 dds, whereas short term are favorable compared to ZE responses, which develop from 1 to 1.5 years under in situ conditions. The percentage of germination of somatic embryos combining kin, ANA, AG3, BA and 2,4-D at 40 and 210 dds, shown in Figure 9, where obviously the medium without the presence of growth regulators had no effect positive in embryonic development; comparing the combination 4.44+1.35uM (BA+2,4-D).

Figure 9 Combination regulators and blackening levels of cultured in vitro responses observed according to their viability tissues to promote somatic embryogene.
Embryonic development and responses associated germination were observed in embryos meristematic cell division in apical region type. Which shows an embryonic indispensable maturation in the conversion process plant (germination). Development related to leaf development and the apparent photosynthetic activity could be observed (Figure 8) structures, which coincides with Litz et al.,7 who reported that the expansion of the first sheet always circinada or after germination.
The production of embryonic structures derived from apical meristem area growth mature somatic embryos from 30 dds was evident (Figure 10), averaging 8.7 and 12.1 somatic embryos under the effect of 1.33+0.45 and 4.44+1.35 (μM) BA+2,4-D, respectively. This, evidenced a recurring somatic embryogenesis (ESR), coinciding with who point out that mature somatic embryos are allowed to form new embryos derived from epidermal cells. However, the 2.48% exhibit deformations in morphology (fan shape), and promptly let this kind of derangement may be related to bipolar endogenous auxin transport by Hiraga et al.20
The combination of 4.44+1.35 uM BA+2,4-D in MS basal medium (1962) with 50% reduction in the source of nitrate, stimulate the formation of potentially embryogenic callus at 170 days of cultivation, which characterized by a creamy color, compact consistency and nodular appearance.MS basal medium (1962) with NH4NO3 and KNO3 reduction at 75 and 25%, respectively stimulated the production of somatic embryos from 30 days; however, maturation occurs not before 100 days after subculturing. The combination of 4.44+1.35 uM BA+2,4-D stimulated responses associated germination of somatic embryos.Tissue maturation in each of the stages of somatic embryogénics cycad, is substantial for somatic embryos with good regeneration potential. The application of activated carbon under a previous nutritional stress, recurrent somatic embryogenesis stimulated somatic embryos more than 220 days after subculture. Finally, in Cycas sp. according to the results obtained, we consider that a limitation were the in vitro responses that are very slow, and the assessment of the each stages, as well as various constituents of the growing medium.21–25
The authors thanks the National Council for Science and Technology for the scholarship 736230/595557 during the course of academic training in the "Master of Science in Agricultural Biotechnology" program.
The authors declare there is no conflict of interest.
None.

©2019 la, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.