Journal of
eISSN: 2572-8466


Review Article Volume 11 Issue 4
Department of Bioengineering, University of California, Los Angeles (UCLA), USA
Correspondence: Dr. Bill Tawil, Department of Bioengineering, UCLA School of Engineering, Los Angeles, CA 90095-1600, USA
Received: July 06, 2024 | Published: July 29, 2024
Citation: Eva S, Bill T. Endometriosis: pathophysiology, market analysis, and research landscape. J Appl Biotechnol Bioeng. 2024;11(4):94-105. DOI: 10.15406/jabb.2024.11.00366
Endometriosis is a chronic gynecological condition affecting millions of women globally, significantly impacting their quality of life and reproductive health. Characterized by the presence of endometrial-like tissue outside the uterus, this condition leads to inflammation, chronic pelvic pain, infertility, and compromised organ function. Despite its prevalence, there is no definitive cure for endometriosis, and current treatments primarily focus on managing symptoms and preserving fertility. Surgical interventions, such as laparoscopic excision, remain central to managing severe cases. The increasing incidence of endometriosis and the limitations of existing treatments have driven substantial research efforts toward more effective therapies, including personalized medicine approaches. The global endometriosis treatment market, valued at approximately USD 1.3 billion in 2022, is projected to reach USD 3.21 billion by 2030, driven by advancements in diagnostics and novel therapeutics.41,29,7 Key market players include AbbVie and Pfizer, with significant contributions from ongoing clinical trials exploring innovative treatments such as Bayer's P2X3 receptor antagonist. This review examines the pathophysiology of endometriosis, evaluates current therapeutic strategies, and highlights emerging research trends, providing a comprehensive perspective on the future of endometriosis management.
Keywords: tissue engineering, chronic pelvic pain, infertility, laparoscopic excision, personalized medicine, diagnostic techniques, therapeutics, clinical trials, pharmaceutical industry, hormonal therapy, surgical interventions, non-invasive diagnostics, biomarkers, inflammatory response, estrogen regulation, novel pharmacological agents, aromatase inhibitors
GnRH, gonadotropin-releasing hormone; NSAIDs, nonsteroidal anti-inflammatory drugs; LH, luteinizing hormone; FSH, follicle-stimulating hormone; COCs, combined oral contraceptives; VEGF, vascular endothelial growth factor MCP-1, monocyte chemotactic protein; NGF, 1 nerve growth factor; PGE2, prostaglandin E2; E2, estradiol; PLGA, poly(lactic-co-glycolic) acid; FDA, food and drug administration; CAGR, compound annual growth rate; USD, United States Dollar
Endometriosis is a prevalent gynecological disorder affecting millions of women worldwide, exerting a significant toll on their quality of life and reproductive health.1 While mild cases of endometriosis may initially present with subtle symptoms or even be asymptomatic, untreated instances can progress to severe complications such as chronic pelvic pain, infertility, and compromised organ function.4,10,24,25 Currently, no definitive cure exists for endometriosis, with treatment options primarily focused on symptom management and fertility preservation.2,10 Surgical intervention, including laparoscopic excision or ablation of endometrial implants, remains a cornerstone in the management of severe cases.38–41
The global endometriosis treatment market was valued at approximately USD 1.3 billion in 2022 and is projected to reach USD 3.21 billion by 2030, with a compound annual growth rate (CAGR) of 13.51%.29 This growth is fueled by increasing awareness, advancements in diagnostic techniques, and the development of novel therapeutics.7 Key players in the market include AbbVie with its product Elagolix (Orilissa), which generated $1.358 billion in sales in 2022, and Pfizer's Relugolix (MYFEMBREE), recently approved by the FDA and projected to reach $900 million in sales by 2032.11 Ongoing clinical trials are exploring innovative treatments and technologies, such as robotic-assisted surgery and novel pharmacological agents. Bayer's phase 2 clinical trial for the highly selective P2X3 receptor antagonist is among the noteworthy ongoing studies aimed at enhancing treatment efficacy and patient outcomes.49–60
Other significant trials include studies on purified fatty acids for endometriosis-associated pain, Letrozole for ovarian stimulation in endometriosis patients, and estetrol/drospirenone for reducing the size of endometriomas.49–60 There is a growing demand for more effective therapies and a shift towards personalized medicine approaches.24 This review aims to explore the pathophysiology of endometriosis, therapeutic strategies ranging from pharmacological interventions to surgical techniques, and the evolving landscape of endometriosis research. By synthesizing current knowledge and emerging trends, this review seeks to provide insights into the complexities of endometriosis management and offer a glimpse into the future of the treatment landscape for this debilitating condition.
Healthy tissue
Endometriosis can attach itself anywhere on the peritoneal surfaces, targeting tissues lining the ovaries, fallopian tubes, and intestines.63 The fallopian tubes are an integral part of the female reproductive system, providing the pathway for ova to travel from the ovaries to the uterus.4–67 The inner lining of the fallopian tubes, known as the endothelium, plays a vital role in this process by facilitating the movement of the ova and providing an optimal environment for fertilization.63–67
Under normal circumstances, the endothelial lining of the fallopian tubes is composed of ciliated and secretory cells, as demonstrated by Figure 8. The ciliated cells have hair-like projections that gently move in a coordinated wave-like manner to propel the ova towards the uterus.63 This movement is essential for the timely transport of the egg, increasing the chances of successful fertilization.63 The secretory cells in the fallopian tubes produce a nutrient-rich fluid that supports both the ova and the sperm, creating a conducive environment for fertilization.63–67 This fluid contains vital proteins and enzymes that assist in the maturation of the sperm and the egg, enhancing the likelihood of conception.64 Additionally, the secretory cells help maintain the pH balance and ionic composition of the tubal environment, which is critical for the survival and function of gametes.64 Furthermore, the endothelial lining exhibits anti-adhesive properties, which are crucial for preventing the attachment of any cells or particles that could obstruct the tubes.63–67 This feature helps to keep the fallopian tubes open and functional, ensuring an unobstructed passage for the egg.65 The endothelium also plays a role in immune defense by producing antimicrobial peptides and other molecules that protect against infections.65
During ovulation, the fallopian tubes respond to hormonal signals by increasing the motility of the cilia and enhancing the secretory activity, optimizing the conditions for egg transport and fertilization.66 The coordinated contraction of the smooth muscles in the fallopian tubes, regulated by the endothelial lining, also aids in the movement of the ova.66 This peristaltic movement is critical in ensuring that the egg reaches the site of fertilization in the ampulla of the fallopian tube.66 Overall, the endothelial lining of the fallopian tubes plays a crucial role in reproductive health, supporting the movement, nourishment, and protection of the ova and sperm. Its proper function is essential for fertility and successful conception.63-67
Pathophysiology
Diseased tissue
Endometriosis is a chronic gynecological condition characterized by the presence of endometrial-like tissue outside the uterus.1–4 As demonstrated in Figure 1A, these lesions significantly impact the functioning of the uterus, leading to inflammation, pain, and infertility.2 The ectopic endometrial-like tissue behaves similarly to the normal endometrium, responding to hormonal changes during the menstrual cycle.24,25 This response includes proliferation, inflammation, and shedding of tissue, which can cause significant pain and the formation of adhesions and scar tissue.3
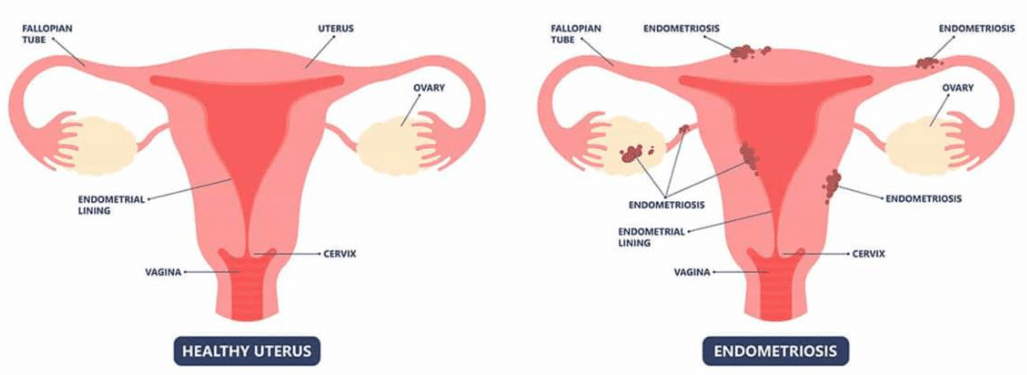
Figure 1a Healthy uterus versus endometriosis.37
The image shows a comparison between a healthy uterus and one affected by endometriosis, highlighting the differences in tissue structure and appearance.
The exact pathogenesis of endometriosis remains uncertain, but several theories have been proposed, including retrograde menstruation, coelomic metaplasia, and embryonic cell rest theories.24,25 The most widely accepted theory is the retrograde menstruation theory, also known as Samson’s theory, which suggests that menstrual blood containing endometrial cells flows backward through the fallopian tubes into the pelvic cavity instead of leaving the body. These displaced endometrial cells adhere to the surfaces of pelvic organs and tissues, where they implant and grow.4,24,25 However, since retrograde menstruation is common among women of reproductive age, other determining factors likely contribute to the development of endometriosis.24,25 The coelomic metaplasia theory proposes that lesions arise from the transformation of cells rather than their migration, suggesting that coelomic epithelium lining the pelvic organs can transform into endometrial-like cells, potentially triggered by environmental factors, hormonal changes, or inflammation. The embryonic cell rest theory suggests that remnants of embryonic cells, which normally differentiate into endometrial cells, persist in the pelvic region and develop into endometriosis later in life.4
There is evidence suggesting a genetic predisposition to endometriosis. First-degree relatives of affected individuals have a higher risk of developing the condition.4,24,25 Genetic studies have identified several susceptibility loci associated with endometriosis, although the exact genetic mechanisms remain unclear.24,25 Epigenetic modifications, such as DNA methylation and histone acetylation, may also play a role in regulating genes involved in endometriosis.4,24,25
Endometriosis is associated with a heightened inflammatory response and immune system dysfunction.4 The presence of ectopic endometrial tissue activates the release of pro-inflammatory cytokines and growth factors, leading to chronic inflammation.3,4 Additionally, immune cells such as macrophages and natural killer cells exhibit altered functions, which may contribute to the survival and growth of ectopic endometrial tissue.3
Estrogen plays a crucial role in the pathophysiology of endometriosis. The ectopic endometrial tissue is highly sensitive to estrogen, which promotes its growth and survival.3,4,24,25 Aromatase, an enzyme responsible for estrogen synthesis, is found in higher levels in endometriotic lesions compared to normal endometrium. This local production of estrogen further stimulates the growth of ectopic tissue.3 Chronic pain in endometriosis is not only due to inflammation but also involves neurological changes.3,4,24,25 Ectopic endometrial lesions can infiltrate nerves, leading to nerve damage and the formation of nerve fibers within the lesions.4 This neuroangiogenesis contributes to the chronic pain experienced by many patients with endometriosis.4
Endometriosis is a condition resulting from tissue, closely resembling the uterine lining, implanting itself onto peritoneal surfaces.1-4 Retrograde transplanted endometrial tissue can develop a blood supply and invade adjacent structures.4 These tissues are infiltrated by sensory, sympathetic, and parasympathetic nerves, which provoke an inflammatory response.4 This process initiates a cascade of biochemical reactions, as demonstrated in Figure 1B.4 Endometriotic implants secrete estradiol (E2) and prostaglandin E2 (PGE2), attracting macrophages (via monocyte chemotactic protein 1 [MCP-1]), neurotrophic factors (such as nerve growth factor [NGF]), tissue remodeling enzymes, and proangiogenic substances like vascular endothelial growth factor (VEGF) and interleukin-8.4 These lesions also release haptoglobin, which decreases macrophage adhesion and phagocytic function.4
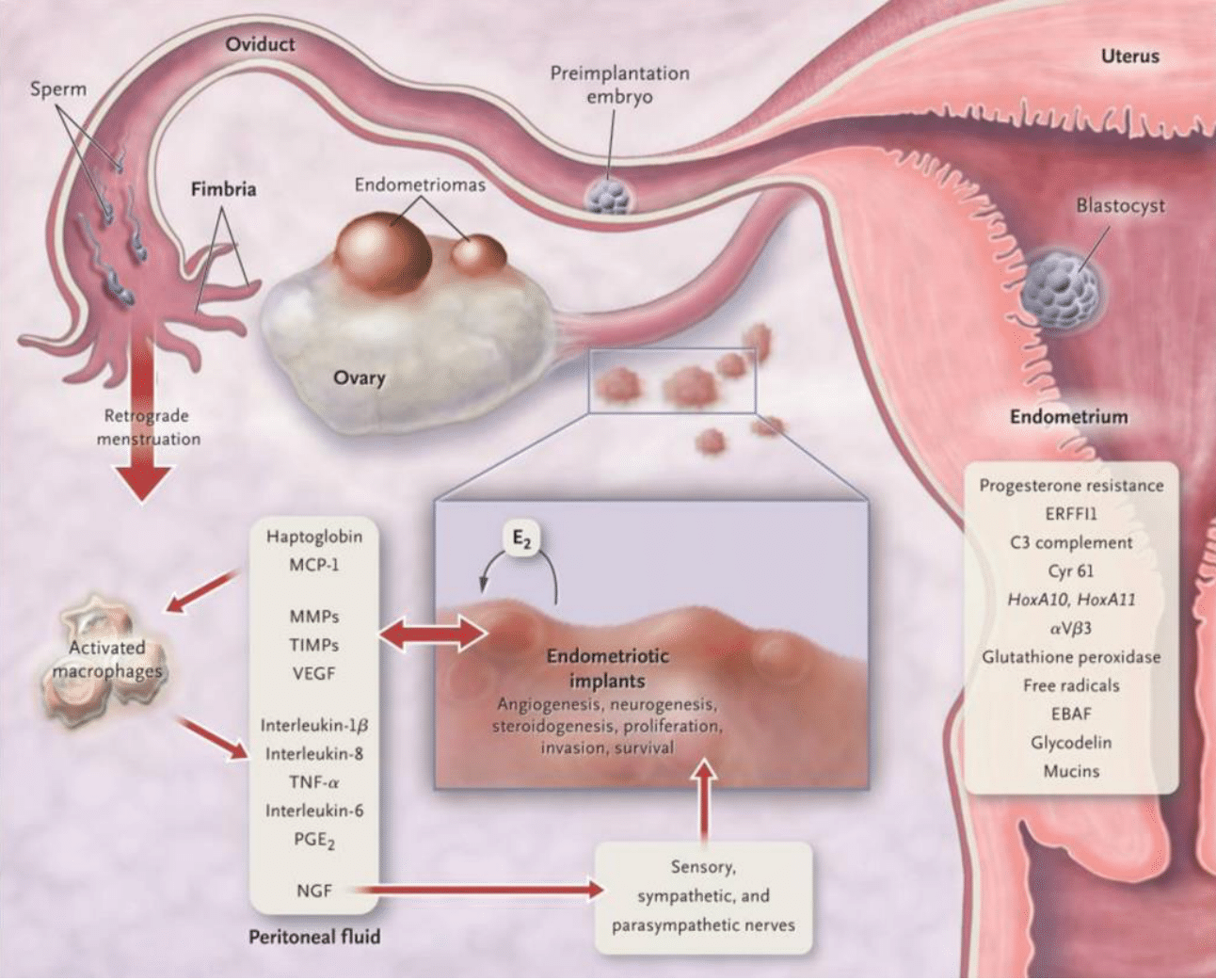
Figure 1b Pathophysiology of pain and infertility associated with endometriosis.4
This image shows the complex pathophysiological mechanisms that lead to pain and infertility in individuals with endometriosis, demonstrating how ectopic endometrial tissue triggers inflammatory responses and disrupts normal reproductive functions.
In women with endometriosis, lesions and activated macrophages in the peritoneal fluid secrete various pro-inflammatory cytokines.4 Both local and systemic estradiol can stimulate prostaglandin production in lesions, activating pain fibers, and enhancing neuronal invasion through increased nerve growth factor, leading to chronic inflammatory pain.4 Ectopic endometrial lesions can infiltrate nerves, causing nerve damage and the formation of nerve fibers within the lesions, contributing to chronic pain.4
As demonstrated in Figure 2, the histology of endometriosis from advanced and early lesions shows the detailed structure of endometrial epithelia, stroma, fibrosis, and inflammation in cynomolgus monkeys.5 These images illustrate the complex cellular environment and inflammatory response associated with endometriosis.5 Figure 2H shows infiltration of mononuclear cells into the lesion, and then T-cells (Figure 2J), as well as CD163-positive macrophages and macrophage-derived foreign body multinucleated giant cells (Figure 2K), can be observed in the same location, which is indicative of the inflammatory response.5 This heightened inflammatory response and abnormal endometrial tissue growth can significantly impact fertility.4,10,24–25 The inflammatory process has toxic effects on gametes and embryos, and the ectopic endometrium is resistant to progesterone, creating a hostile environment for embryonic implantation.4,10,24–25 Genes such as HoxA10, HoxA11, and the αVβ3 integrin are not upregulated by progesterone in the ectopic endometrium, making it unsuitable for embryo implantation.4 Endocrine-disrupting chemicals may enhance progesterone resistance and potentially cause immune dysfunction.4
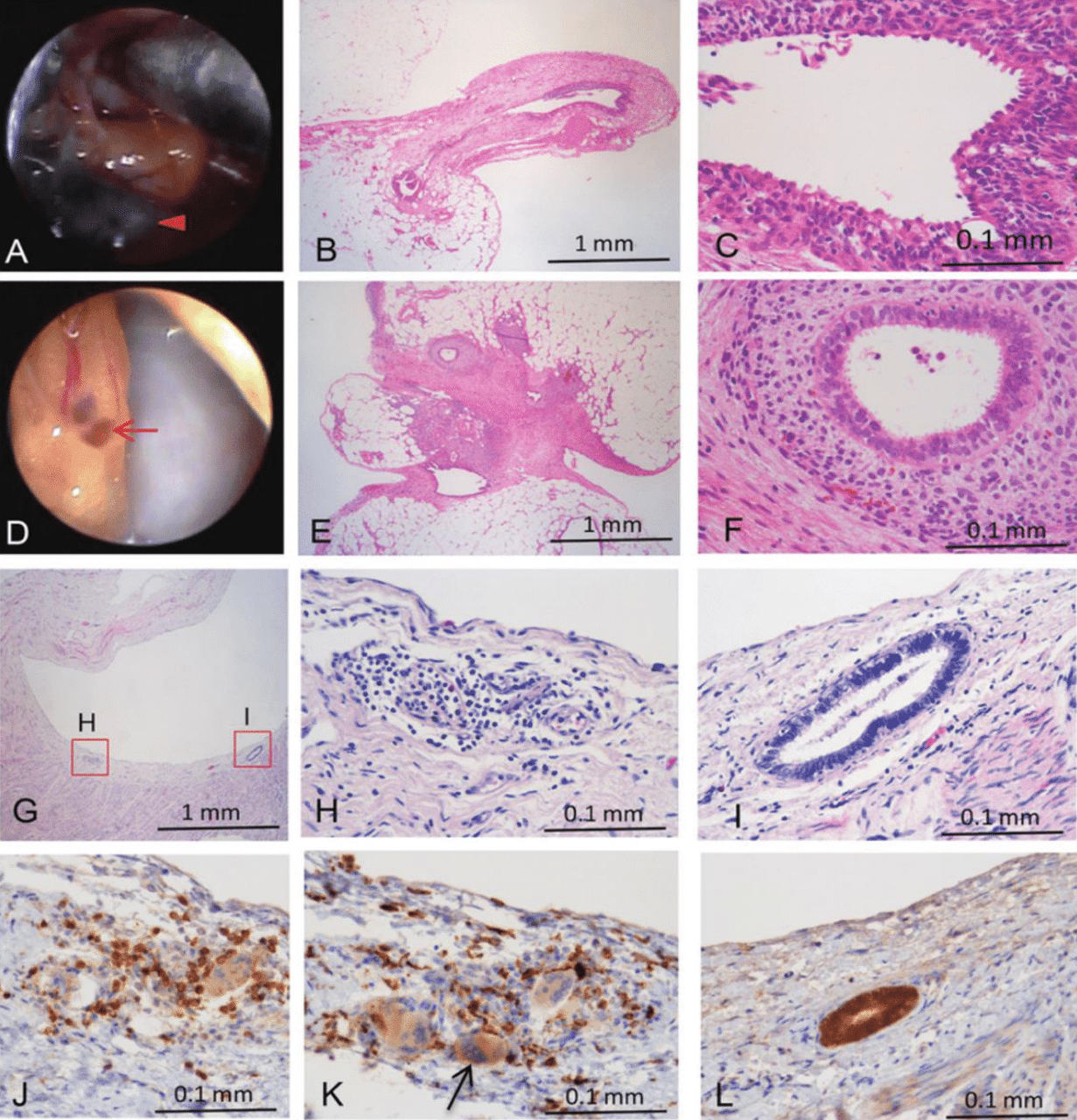
Figure 2 Histology of endometriosis from an advanced lesion and an early lesion in cynomolgus monkeys.5
The series of images shows the histological examination of endometriosis at various stages in cynomolgus monkeys. It includes detailed views of cysts, vesicles, and endometrial glands, highlighting cellular changes, fibrosis, and inflammatory responses at different magnifications. Specifically, the legend enumeration is as follows: A) Stationary image of a cyst. B) Endometrial epithelia, endometrial stroma, and fibrosis in the cyst (× 40 magnification). C)Higher magnification of the cyst showing detailed endometrial epithelia, stroma, and fibrosis (× 400 magnification). D) Stationary picture of a vesicle. E) Endometrial epithelia, endometrial stroma, and fibrosis in the vesicle (× 40 magnification). F) Higher magnification of the vesicle showing detailed endometrial epithelia, stroma, and fibrosis (× 400 magnification). G) Inflammation and an endometrial gland in the peritoneum of an early lesion from monkey #7 (× 40 magnification). H) Higher magnification of the early lesion showing infiltration of mononuclear cells (× 400 magnification). I) Higher magnification of the early lesion showing an endometrial gland (× 400 magnification). J) CD3-positive lymphocytes (T cells) in the same location as Figure 2H in a serial section (× 400 magnification). K) CD163-positive macrophages and macrophage-derived foreign body multinucleated giant cells in the same location as Figure 2H in a serial section (× 400 magnification). L) Expression of estrogen receptor alpha in an endometrial gland in the same location as Figure 2I in a serial section (× 400 magnification).
Market analysis
Overview
Endometriosis is a significant global health issue, affecting approximately 10% of women of reproductive age, which translates to around 176 million women worldwide.2 In the United States, it is estimated that about 6.5 million women suffer from this condition.2 The economic burden of endometriosis is substantial due to both direct healthcare costs and indirect costs such as lost productivity.3
The market for endometriosis treatments is driven by the high prevalence of the condition and the chronic nature of its symptoms, which require long-term management.31 Currently, there is no definitive cure for endometriosis, and treatments focus primarily on managing pain and reducing the growth of endometrial tissue.31 The treatment landscape includes pharmacological interventions such as nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapies (e.g., oral contraceptives, GnRH agonists and antagonists, and progestins), and surgical options.30–49
As demonstrated in Figure 3, the endometriosis treatment market is expected to see continued expansion.6 The global endometriosis treatment market size was valued at approximately USD 1.3 billion in 2022 and is projected to reach USD 3.21 billion by 2030, with a compound annual growth rate (CAGR) of 13.51%.6 Key factors contributing to this growth include increasing awareness of the condition, advancements in diagnostic techniques, and the development of novel therapeutics.6-9
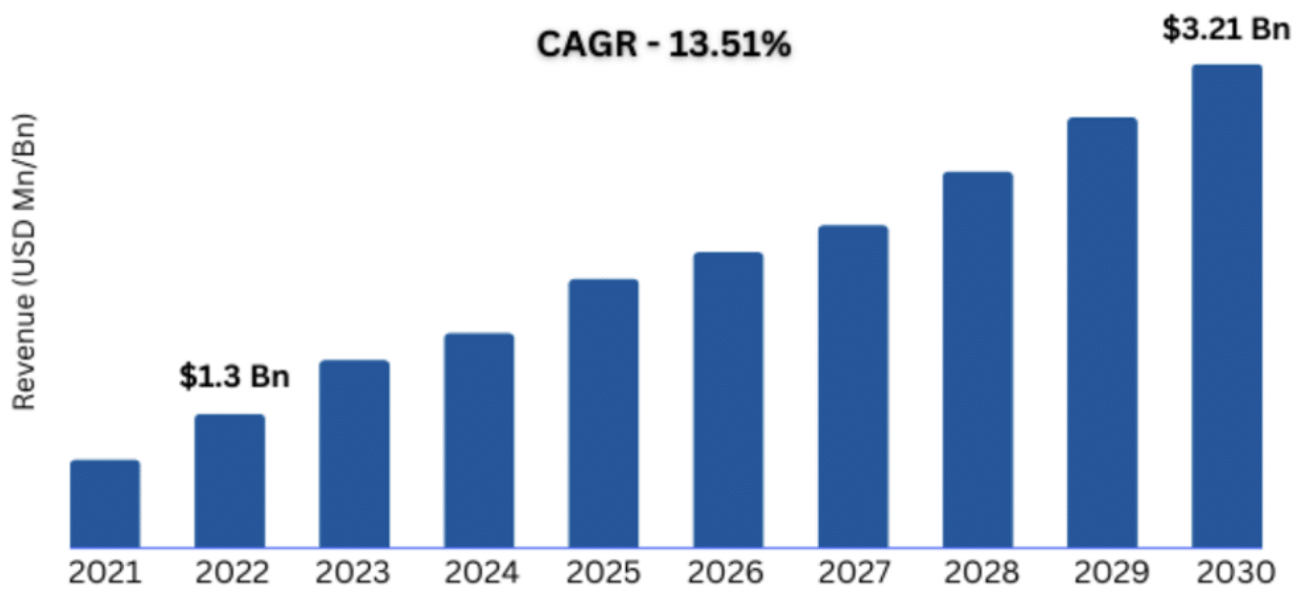
Figure 3 Global endometriosis treatment market size.6
This image shows the global market size for endometriosis treatments, demonstrating an emerging trend for market increase.
Regional analysis
The regional distribution of revenue from the endometriosis treatment market reflects varying levels of healthcare access, awareness, and economic development7. North America holds the largest market share, driven by the high prevalence of endometriosis, advanced healthcare infrastructure, and significant investment in research and development.7–9 As demonstrated in Figure 4, North America held a 48.7% revenue share in the market of endometriosis treatment in 2022.7 In the United States, the increasing number of women diagnosed with endometriosis and the availability of advanced treatments contribute to the robust market growth.7,9

Figure 4 Endometriosis treatment market, trends by region.7
The image demonstrates the trends in the endometriosis treatment market across different geographical regions, illustrating regional variations in market growth, and the largest market.
Europe follows closely behind North America in terms of market share,8 with countries like Germany, the United Kingdom, and France leading endometriosis research.8 The European market benefits from strong healthcare systems and widespread access to medical care, which supports early diagnosis and comprehensive treatment plans.8 The Asia-Pacific region is expected to witness the fastest growth during the forecast period,9 as reflected by Figure 4. This growth is attributed to rising awareness about endometriosis, improving healthcare infrastructure, and increasing healthcare expenditure in countries such as China, Japan, and India.9 Latin America, the Middle East, and Africa regions also present significant growth opportunities, with the key challenges being limited awareness and access to healthcare services9. However, ongoing efforts to improve healthcare infrastructure and increase awareness about endometriosis are expected to boost market growth in the coming years.9
Overall, the endometriosis treatment market is headed for substantial growth globally, with the growing focus on women's health and the introduction of new treatment options being the main driving force behind the market expansion.6–9
Existing treatment options
Endometriosis is a chronic condition with no definitive cure, necessitating a multifaceted approach to treatment that includes pharmacological therapies, surgical interventions, and diagnostic measures.10,30–31 The goal of treatment is to manage pain, reduce endometrial growths, and address infertility issues.2,10,30–32 The choice of treatment depends on the severity of the symptoms, the patient's age, and their desire for fertility preservation.32
As demonstrated by Figure 5, U.S. endometriosis treatment market can be divided into pain medication and hormone therapy (Figure 9C), with pain medication constituting a smaller share.7 The majority of the pain management medication for endometriosis falls into the nonsteroidal anti-inflammatory drugs (NSAIDs) category.30 NSAIDs, such as ibuprofen, are commonly used to manage pain and inflammation associated with endometriosis.30 These drugs work by inhibiting the cyclooxygenase (COX) enzymes, COX-1 and COX-2, which are involved in the production of prostaglandins that cause inflammation and pain.30,31 NSAIDs are often the first line of treatment due to their effectiveness and over-the-counter availability, but their long-term use can lead to gastrointestinal and renal toxicity.30,31
U.S. endometriosis treatment market has reported a revenue of over a half billion dollars in 2021, with projected robust market growth (Figure 5). Hormone therapy constitutes a major share of the market.7 Hormone therapies can be divided into multiple sub-groups, differing by pharmacology, treatment efficacy, and side effects. Hormone therapy targeted specifically towards endometriosis can be classified into GnRH agonists, GnRH antagonists, progestins, combined oral contraceptives, aromatase inhibitors, and androgens.13–31
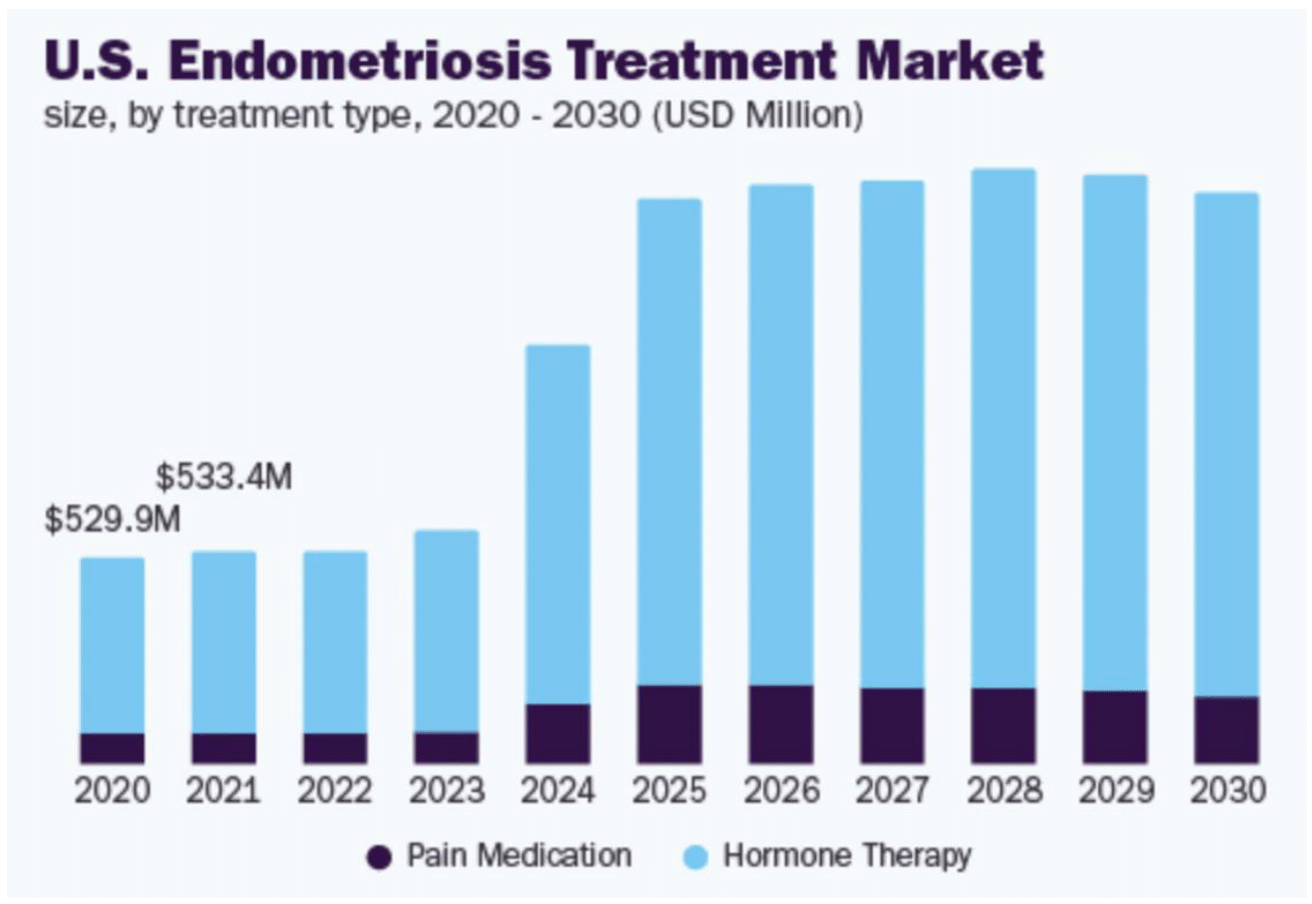
Figure 5 U.S. Endometriosis treatment market size by treatment type, 2020-2030.7
This figure shows projected market size data for endometriosis treatments in the United States, segmented by treatment type from 2020 to 2030.
To begin with, GnRH agonists, such as leuprolide acetate, are used to suppress the production of estrogen, which fuels the growth of endometrial tissue.32 These drugs work by downregulating the GnRH receptors in the pituitary gland, leading to decreased secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and subsequently lowering estrogen levels.32,33 However, GnRH agonists can cause side effects such as bone density loss, hot flashes, and mood changes, which may limit their long-term use.32,34
On the other hand, newer GnRH antagonists such as Elagolix directly inhibit GnRH receptors, resulting in a rapid decrease in LH and FSH levels and reducing estrogen production.30,35 Elagolix has shown high efficacy in reducing endometriosis pain, as indicated by its market share growth.30,35–36 Unlike GnRH agonists, elagolix allows for partial estrogen suppression, which can reduce some of the severe side effects associated with complete estrogen deprivation, such as bone density loss.30,35–37 However, patients may still experience hot flashes, headaches, and nausea.35,36
Another prominent form of hormone therapy is progestins.30,38–39 Progestins, such as medroxyprogesterone acetate, are synthetic hormones that suppress ovulation and reduce the growth of endometrial tissue by counteracting estrogen’s effects.38 These drugs are effective in managing pain and reducing lesion size but can cause side effects such as weight gain, mood changes, and bone density loss with long-term use.30,38–39
Some versatile hormonal therapies, not targeted specifically towards endometriosis, have moderate to high efficacy for endometriosis symptom management.30 For instance, combined oral contraceptives (COCs), such as ethinyl estradiol/norethindrone, are often used to suppress ovulation and stabilize endometrial tissue, reducing menstrual flow and pain39. These contraceptives are commonly prescribed for dysmenorrhea and provide moderate efficacy in managing endometriosis symptoms.31,39 However, they carry risks of side effects such as nausea, weight gain, and an increased risk of thrombosis.31,39
Another prominent form of hormonal therapy is aromatase inhibitors.40,41 They inhibit the enzyme aromatase, which is responsible for converting androgens to estrogen.40 By reducing estrogen production, these drugs can decrease the growth of endometrial tissue.40,41 Aromatase inhibitors are often used in combination with other hormonal therapies to enhance their effectiveness.41 While they are generally well-tolerated, potential side effects include bone density loss, joint pain, and fatigue.41
Finally, the last category of common hormonal therapy for endometriosis is androgens, such as danazol. Danazol a synthetic steroid that inhibits gonadotropins and reduces estrogen levels.42,43 Although effective, its use has declined due to significant side effects such as weight gain, acne, and liver toxicity.42,43
The summary of the existing pharmacological treatment options is reflected by Table 1. However, for patients who do not respond to pharmacological treatments or have severe endometriosis surgical interventions are often considered.15 The primary goals of surgery are to remove or destroy endometrial lesions, relieve pain, and improve fertility outcomes.15 The surgical methods can be divided into three main categories: laparoscopy, laparotomy, and hysterectomy.
|
Symptom addressed |
Medication category |
Medication name |
Mechanism of action |
Toxicity |
Efficacy for symptom management |
|
Pain and Inflammation |
NSAIDs |
Ibuprofen |
Inhibits COX-1 and COX-2 enzymes, reducing prostaglandins30,31,32 |
||
|
Hormonal regulation |
GnRH Agonists |
Leuprolide acetate |
Suppresses estrogen production by downregulating GnRH receptors30,33,34 |
||
|
Hormonal regulation |
GnRH Antagonists |
Elagolix (Orilissa) |
Inhibits GnRH receptors, reducing estrogen production30,35,36 |
||
|
Pain and Inflammation |
Progestins |
Medroxy progesterone acetate (DepoProvera) |
High efficacy for pain and lesion reduction32 |
||
|
Hormonal regulation |
Combined Oral Contraceptives |
Ethinyl estradiol/ norethindrone |
Moderate efficacy, especially for dysmenorrhea31 |
||
|
Hormonal regulation |
Aromatase Inhibitors |
Letrozole |
Moderate efficacy, often used with other therapies32 |
||
|
Pain and Inflammation |
Androgens |
Danazol |
Suppresses ovarian function and reduces estrogen levels30,43 |
Moderate to high efficacy, often used as second-line therapy30,43 |
To begin with, laparoscopy is the gold standard for both diagnosing and treating endometriosis.15 This minimally invasive procedure allows surgeons to visualize the pelvic cavity and excise or ablate endometrial lesions.15 Laparoscopic excision has been shown to provide significant pain relief and improve fertility in many patients.15 However, endometriosis can recur, necessitating repeat surgeries for some individuals.15
In more severe cases, a laparotomy, which is an open surgical procedure, may be required to remove extensive endometrial growths and adhesions.15 This approach is less common due to its invasive nature and longer recovery times compared to laparoscopy.15 Nevertheless, it remains an option for patients with extensive disease involvement that cannot be adequately addressed through laparoscopy.15
For women with severe, refractory endometriosis who do not wish to preserve fertility, a hysterectomy (removal of the uterus) with or without oophorectomy (removal of the ovaries) may be considered.15 This procedure can provide definitive pain relief by removing the primary source of endometrial tissue.15 However, it is a major surgery with significant implications for the patient's hormonal balance and overall health.15
Overall, laparoscopy and laparotomy are the only available treatment options that give patients a beacon of hope in fertility restoration, however, in a lot of cases endometriosis can recur.10,15 For severe cases, when the patient does not wish to preserve fertility, hysterectomy is considered to significantly reduce the chance of recurrence of the disease.15 The summary of the existing surgical interventions for endometriosis treatment is reflected in Table 2.
|
Procedure |
Method |
Advantages |
Limitations |
|
Laparoscopy |
- Small incisions; use of a laparoscope to excise or ablate lesions38 |
- Minimally invasive; shorter recovery time38 |
- Recurrence of lesions possible38 |
|
Laparotomy |
- Large abdominal incision; excision of deep infiltrating endometriosis39 |
- Effective for extensive disease39 |
- Longer recovery; increased risk of complications39 |
|
Hysterectomy |
- Removal of uterus (with or without ovaries)40 |
- Definitive solution for severe symptoms; eliminates pain40 |
- Infertility; major surgery with hormonal implications40 |
|
Robot-assisted laparoscopy |
- Use of robotic systems for precise excision of lesions41 |
- Enhanced precision; minimally invasive41 |
- High cost; requires specialized training41 |
Table 2 Existing surgical procedures for endometriosis treatment.38–41
Another crucial segment of the endometriosis market is diagnostics. Accurate diagnosis of endometriosis is integral for effective management10. The current standard for definitive diagnosis is laparoscopy, which allows direct visualization and biopsy of endometrial lesions.15 However, non-invasive diagnostic methods are under development to reduce the need for surgical diagnosis.15 For instance, imaging techniques such as transvaginal ultrasound and magnetic resonance imaging (MRI) can help identify endometriomas and deep infiltrating endometriosis.15 While these methods are not definitive, they can guide clinical suspicion and aid in preoperative planning.15 Research is ongoing to identify reliable biomarkers for endometriosis that can be detected through blood tests or other non-invasive means.15 Potential biomarkers include CA-125, a protein that is elevated in some women with endometriosis, although it is not specific to the condition and has limited diagnostic accuracy.16,15
According to Figure 6, the largest share of medication distribution for endometriosis treatments is from retail pharmacies, which reflects the accessibility and common use of pharmacological treatments.15,7 However, as surgical treatments become more accessible, efficient, and less invasive, there may be an increase in the distribution share from hospital pharmacies.7-9,15
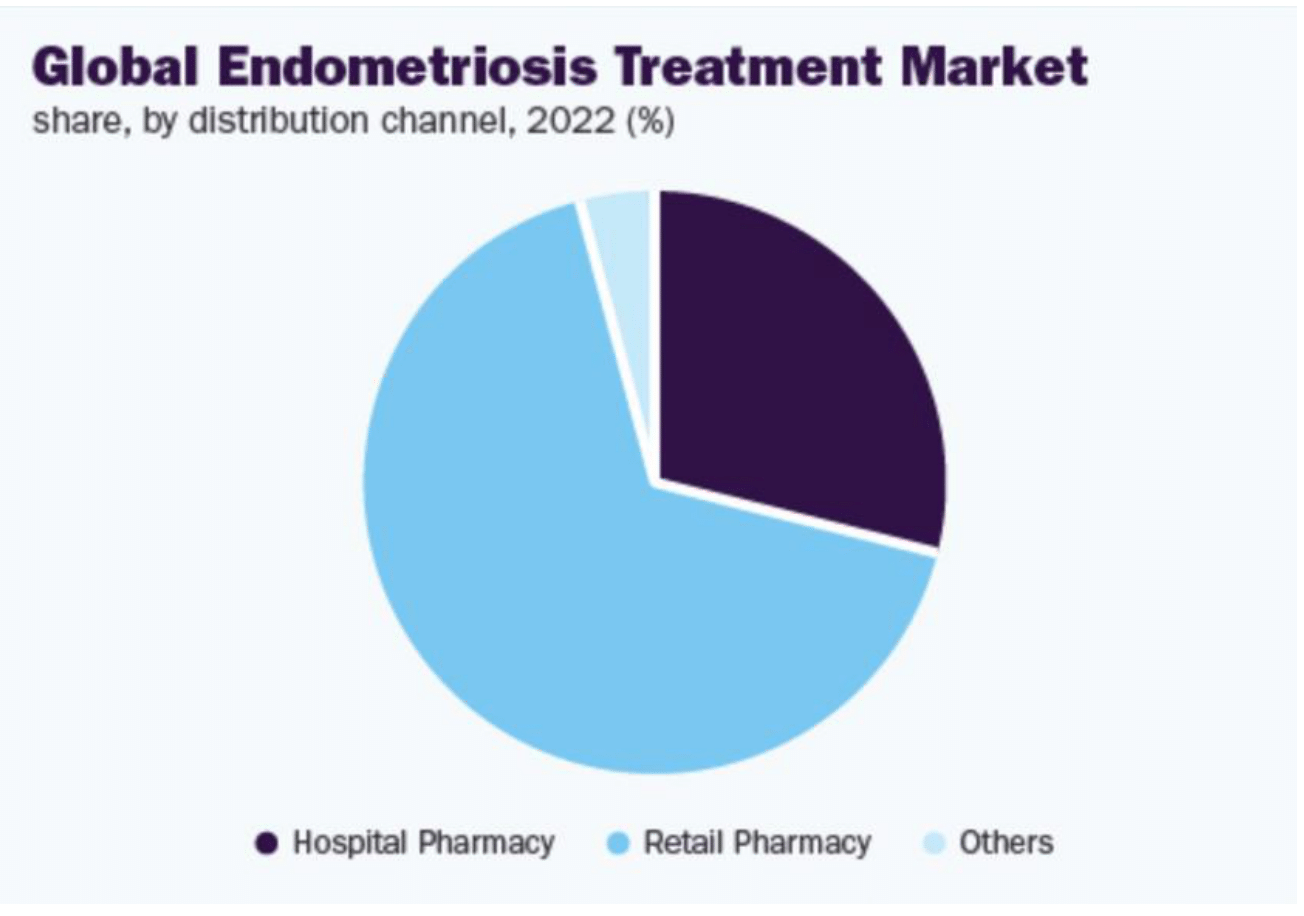
Figure 6 Global endometriosis treatment market share by distribution channel, 2022.7
The image shows the market share distribution for endometriosis treatments globally by distribution channel in 2022.
In conclusion, the management of endometriosis involves a combination of pharmacological treatments, surgical interventions, and diagnostic measures.10,12-16,30–44 As research continues to advance, new therapies and diagnostic tools hold promise for improving the quality of life for women with this challenging condition.10,15
Key contributors to the market
The endometriosis treatment market features several prominent players, each significantly impacting the development, production, and commercialization of various therapeutic options. These companies are deeply involved in research and development (R&D), strategic partnerships, and market expansion to address the unmet needs in endometriosis treatment.6–9 Below are some notable contributors and their respective impacts on the market.
ObsEva, in collaboration with Kissei Pharma, has developed Linzagolix, a GnRH receptor antagonist.13 Linzagolix has shown promise in clinical trials for managing endometriosis-associated pain.13 The drug is projected to achieve sales of $150 million in 2024 and $255 million by 2032, with a CAGR of 6.2%.13 However, ObsEva has had its stocks delisted, so it is not obligated to report its revenue, which is reflected in Table 3.59 This development highlights the challenges smaller biotech companies face, even when bringing innovative therapies to market.56
|
Manufacturer |
Drug |
Description |
Sales reported |
Sales estimate 2024 |
Sales estimate2032 |
%CAGR |
|
ObsEva (licensed from Kissei Pharma) |
Linzagolix |
GnRH receptor antagonist |
- |
$150M13 |
$255M13 |
6.2%13 |
|
AbbVie |
Elagolix (Orilissa) |
GnRH receptor antagonist |
$1.358B57 (2022) |
$657M56 (UF, 2024) |
$1.3B13 |
7.8%13 |
|
Pfizer Inc. |
Relugolix (MYFEMBREE) |
GnRH receptor antagonist with estradiol added to reduce bone resorption |
$21M60,66 (2022) |
$458M56 combined (UF, 2024) |
8.5%66 |
|
|
Myovant Sciences |
Relugolix (ORGOVYX) |
GnRH receptor antagonist |
$128M66 (2022) |
$1.1B66 |
9.0%66 |
|
|
AbbVie |
Leuprolide acetate (Lupron Depot) |
GnRH receptor antagonist |
$2.5B65 (2022) |
$2.8B65 (2024) |
$1.1B65 |
4.5%65 |
|
TerSera Therapeutics, AstraZeneca |
Goserelin acetate implant (Zoladex) |
GnRH receptor agonist |
$986m68 (2023) |
$1.004B65 (2024) |
$1.17B65 (2024) |
2.3%65 |
|
Bayer, Teva Pharmaceuticals |
Danocrine (Danazol) |
Synthetic steroid, inhibits gonadotropins |
- |
$100M65 (2024) |
$140M65 -2032 |
3.5%65 |
Table 3 Endometriosis market analysis, breakdown by companies.56–68
As demonstrated by Figure 7, AbbVie is a major player in the endometriosis treatment market. Its notable product Elagolix (Orilissa) is a GnRH receptor antagonist.57 Orilissa was the first oral GnRH antagonist approved for the treatment of moderate to severe pain associated with endometriosis.57 In 2022, Elagolix generated $1.358 billion in sales.62 The projected sales for 2024 are $700 million, expected to grow to $1.3 billion by 2032, with a CAGR of 7.8%.62 AbbVie's significant investment in R&D and extensive clinical trials have solidified its position as a leader in the endometriosis therapeutic market.57
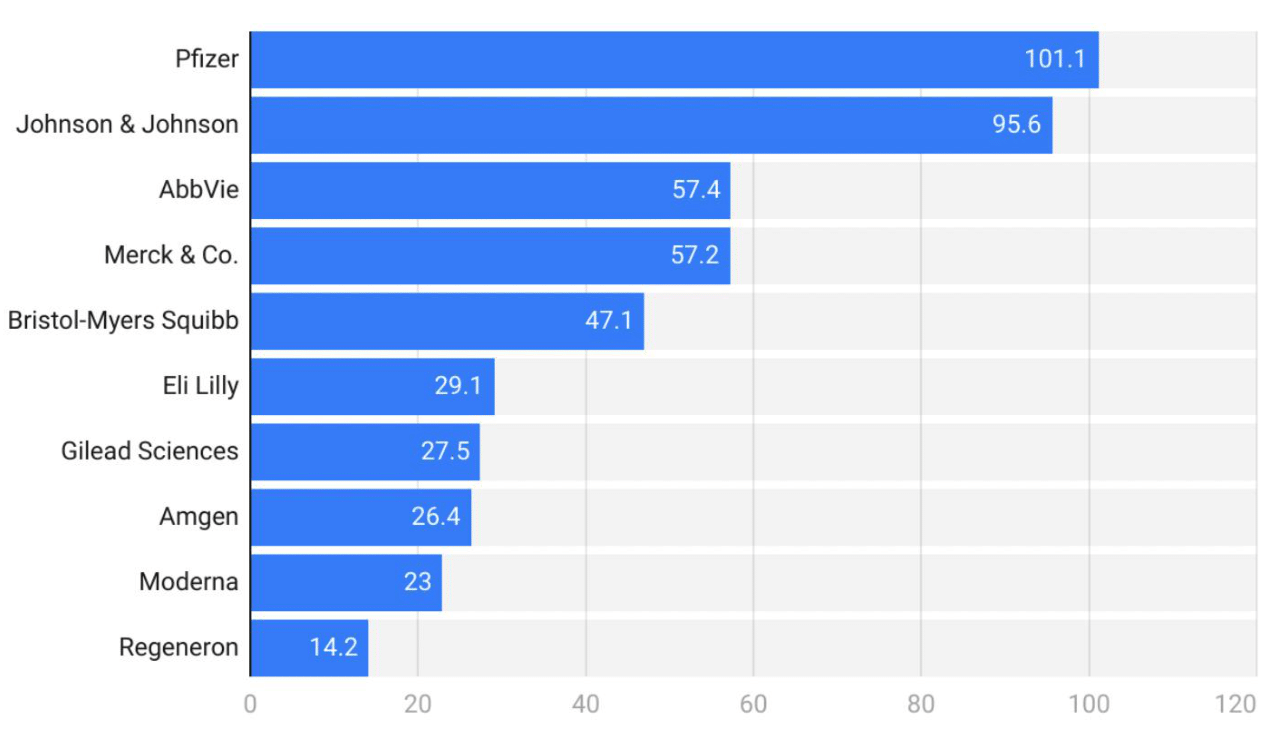
Figure 7 U.S. Pharmaceutical industry statistics by total revenue (USD Billion), breakdown by company.11s
The image presents the total revenue of the U.S. pharmaceutical industry, broken down by company.
Pfizer, in collaboration with Myovant Sciences, developed Relugolix (MYFEMBREE), a GnRH receptor antagonist combined with estradiol and norethindrone acetate to reduce bone resorption.58,60 This combination therapy is designed to manage pain associated with endometriosis while mitigating adverse effects on bone density.58,60 MYFEMBREE is the most recent product in the endometriosis market, gaining FDA approval in august 2022. In 2022, MYFEMBREE reported sales of $21 million.60,66 The sales estimate for 2024 is $400 million, expected to rise to $900 million by 2032, with a CAGR of 8.5%.66 Pfizer’s strategic focus on combination therapies highlights its commitment to comprehensive patient care.58
Myovant Sciences has also developed Relugolix (ORGOVYX), a GnRH receptor antagonist primarily used for prostate cancer but also showing efficacy in managing endometriosis symptoms.60,66 ORGOVYX generated $128 million in sales in 2022, with projections reaching $450 million in 2024 and $1.1 billion by 2032, with a CAGR of 9.0%.66 Myovant Sciences' focus on innovative treatments for hormone-sensitive conditions underscores its vital role in the endometriosis treatment landscape.60
AbbVie’s Lupron Depot, a GnRH receptor agonist, remains a cornerstone in endometriosis management.63 Despite being a well-established treatment, it continues to generate substantial sales, with $800 million reported in 2022.65 The sales are expected to reach $800 million in 2024 and maintain a steady growth rate with a CAGR of 4.5%.65 Lupron Depot’s long-standing efficacy and safety profile have made it a trusted option for many healthcare providers and patients.63
Goserelin acetate, marketed as Zoladex by TerSera Therapeutics and AstraZeneca, is another GnRH receptor agonist used in the treatment of endometriosis.63 It reported sales of $300 million in 2023, with an expected increase to $300 million in 2024 and a CAGR of 4.0%, leading to $420 million by 2032.65 Zoladex is also marketed for prostate cancer, which contributes to its revenue stream.63 This product underscores the ongoing relevance of GnRH agonists in managing endometriosis, particularly in cases where newer therapies may not be suitable.63
Danazol, marketed as Danocrine by Bayer and Teva Pharmaceuticals, is a synthetic steroid that inhibits gonadotropins, thereby reducing estrogen production.65 Although not as commonly used today due to its side effect profile, it still holds a place in the therapeutic arsenal for endometriosis.65 Sales figures for danazol specifically are not prominently reported in financial disclosures or pharmaceutical market analyses, as reflected by Table 3, primarily because it is not a high-revenue drug.65 However, the market for danazol remains steady due to its use in treating chronic conditions like endometriosis.65 The sales estimate for Danazol in 2024 is $100 million, expected to rise to $140 million by 2032, with a CAGR of 3.5%65
In conclusion, the key contributors to the endometriosis treatment market are engaged in a dynamic landscape marked by significant investment in R&D, strategic collaborations, and innovative therapeutic approaches. These efforts are crucial in addressing the complex and multifaceted nature of endometriosis, ultimately improving patient outcomes and quality of life.
Research landscape
The research landscape for endometriosis includes various innovative studies and clinical trials aiming to improve diagnosis and treatment. The studies summarized in Table 4 have paved the way for many ongoing clinical trials and novel treatments. Most notably, Bayer’s first phase of clinical trials for highly selective P2X3 receptor antagonist have successfully concluded, and they are currently in phase 2 of their clinical trial.53
|
Drug/Approach |
Drug class |
Assumed mechanism |
Application |
Comparator(s) |
Primary outcome |
Study phase |
Estimated trial completion |
|
Acupuncture |
Improve blood flow and Qi activation |
Pain relief |
Acupunture needles |
30 min treatment |
Pain reduction measured by visual analog scale (VAS) |
n/a |
04/2020 |
|
Anakira |
IL-1 antagonist |
Anti-inflammatory |
Subcutaneous injections |
Ankira 300mg Placebo |
Change in dysmenorrhea measured by the Biberoglu and Behrman (B&B) score |
1 |
TBD |
|
Botulinum toxin |
Neurotoxic protein from Clostridium botulinum |
Inhibition of acetylcholine release from axons |
Intermuscular injections |
Botulinum toxin A vs Placebo |
Improvement in pain (binary) |
1/2 |
12/2019 |
|
Cabergoline |
Dopamine agonist |
Anti-angiogenic |
Oral |
Cabergoline 0.5mg Placebo |
Change in pain measured by Brief Pain Inventory Interference Scale |
2 |
02/2023 |
|
DLBS1442 |
Bioactive fraction of Phaleria macrocarpa |
Apoptotic Anti-inflammatory Anti-angiogenic |
Oral |
DLBS 100mg, 200mg Mfemanic acid 500 tds |
Reduction in composite pain (VAS) |
2/3 |
05/2020 |
|
Epigallocatechin Gallute (EGCG) |
Polyphenol (Green tea extract) |
Anti-angiogenic |
Oral |
EGCG 400mg bd Placebo |
Change in endometriotic lesion size |
p 2 |
12/2020 |
|
Interleukin-1 Receptor-Associated Kinase-4 (IRAK-4) Inhibitor |
Inhibition of Toll-like receptor/IL-1 receptor complex |
Reduction in expression of inflammatory genes in immune cells |
TBD |
TBD |
TBD |
1 |
TBD |
|
Melatonin |
Neurohormone |
Analgesic Anto-oxidant Anti0inflammatory |
Oral |
Melatonin at 10mg, 20mg Placebo |
Pain reduction (numeric rating scale) |
2 |
02/2021 |
|
Micronized palmitoylethanolamide (PEA)-Transpolydatin |
Endogenous fatty acid amide |
Anti-inflammatory by inhibition of mast cell degranulation |
Oral/sublingual |
Palmitoylethanolamide 600 mg bd sl Palmitoylethanolamide 400 mg and polydatin 40mg bd po |
Change in pelic pain (VAS) |
n/a |
TBD |
|
MT-2990 |
Antibody |
Anti-inflammatory |
Intravenous injections |
MT-2990 Placebo |
Mean change in non-menstual pain |
2 |
05/2020 |
|
P2X3 Antagonist (BAY 181708) |
ATP-gated channel antagonist |
Direct inhibition effect nerve signaling |
Oral |
TBD |
TBD |
1/2 |
Phase 1 completed |
|
P2X3 Antagonist (Gefaxipant) |
ATP-gated channel antagonist |
Direct inhibition effect nerve signaling |
Oral |
Placebo |
Change from baseline in average daily pelvic pain score Adverse events Treatment discontinuations |
2 |
05/2020 |
|
P2X4 Antagonist |
ATP-gated channel antagonist |
Immune cell-mediated inhibition of nerve signaling |
TBD |
TBD |
TBD |
1 |
TBD |
|
Quinagolide |
Dopamine 2-receptor agonist |
Anti-angiogenic Anti-inflammatory |
Vaginal ring |
Quinagolide at 360 mcg, 520 or 1080mcg Placebo |
Change in the mean daily Numerical Rating Scale (NRS) compared to baseline for the worst endometriosis related pain |
2 |
04/2022 |
|
Tetrahydrocannabinol (THC) |
Cannabinoid |
Validated TRPV1 receptor |
Inhaler |
Delta-9-tetrahydrocannabinol 2,7 mg, Cannabidiol 2,5 mg of 1 to 12 puffs |
Pressure threshold in hypogastrium that induces pain |
2 |
07/2019 |
Table 4 Summary of clinical trials as of August 1, 2019
Ongoing clinical trials
Several clinical trials are investigating new treatments and technologies for endometriosis. For instance, a prospective randomized controlled trial by the Mayo Clinic Scottsdale is comparing robotic-assisted versus conventional laparoscopy for the treatment of endometriosis.28 Another trial, the AMY109EU (ACERS) study, is evaluating the safety and effectiveness of an antibody called AMY109, which targets interleukin-8 to reduce inflammation in endometriosis (Table 5).29
|
Trial |
Sponsor/Location |
Phase |
Objective |
Outcome measures |
|
Eliapixant (BAY 1817080) |
Bayer |
Phase 2 |
Evaluate safety and tolerability of the drug, which is a highly selective P2X3 receptor antagonist28,62 |
|
|
AMY109EU antibody |
Chugai Pharmaceutical Co Ltd. |
Phase 1 |
Evaluating the safety and effectiveness of an antibody AMY10927 |
Pain reduction, lesion size reduction27 |
|
IntraVag |
Gynica, Florence, Italy |
Phase 1 |
Evaluate safety and tolerability of cannabinoid-based formulations for endometriosis-associated pain52 |
Safety, tolerability, pharmacokinetics52 |
|
Exosome Therapy |
University of California, San Francisco (UCSF) |
Phase 2 |
Evaluate exosome therapy for drug delivery in endometriosis53 |
Pain reduction, lesion size reduction, adverse events53 |
|
Dichloroacetate (DCA) |
University of Aberdeen, Scotland |
Phase 2 |
Assess efficacy of DCA for reducing endometriosis-associated pain54 |
Pain reduction, need for painkillers54 |
|
ESPriT |
University of Edinburgh |
Phase 2 |
Feasibility of laparoscopic treatment for isolated superficial peritoneal lesions55 |
Pain management, recurrence rates55 |
|
PRE-EMPT |
University of Edinburgh |
Phase 3 |
Compare medical treatments to prevent recurrence after surgical removal56 |
Recurrence rates, pain scores56 |
|
PurFECT |
University of Edinburgh |
Phase 2 |
Efficacy of purified fatty acids for endometriosis-associated pain57 |
Pain reduction, quality of life measures57 |
|
Letrozole Use in Ovarian Stimulation |
UCSF, San Francisco, USA |
Phase 2 |
Evaluate letrozole during ovarian hyperstimulation for endometriosis58 |
Symptom management, embryo quality, pregnancy rates58 |
|
Estetrol/Drospirenone |
Various Locations, USA |
Phase 2 |
Reduce the average size of endometriomas with estetrol/drospirenone58 |
Change in endometrioma volume at 6 months and 3 months58 |
|
NBI-98854 |
Mayo Clinic, Rochester |
Phase 2 |
Investigate non-hormonal treatment for endometriosis-associated pain59 |
Pain reduction, adverse events59 |
|
Immunotherapy |
Yantai Yuhuangding Hospital, China |
Phase 1 |
Assess immunotherapy's effect on endometriosis lesion development60 |
Immune response markers, lesion size, adverse events60 |
Table 5 Summary of Ongoing Clinical Trials as of May 18, 202449–60
Current hormonal treatments for endometriosis include progestins, estroprogestins, GnRH agonists, and GnRH antagonists. While effective, these treatments often come with side effects that limit their long-term use. New delivery methods, such as vaginal rings, implants and intrauterine systems, are being investigated to improve efficacy and reduce side effects.47,55
Non-hormonal treatments are also under investigation. Anakira, an IL-1 antagonist, is being studied for its potential to reduce dysmenorrhea in endometriosis patients.16 Similarly, cabergoline, a dopamine agonist with anti-angiogenic properties, is in Phase 2 trials to evaluate its effectiveness in reducing pain.47 Innovative therapies, such as the use of nanoparticles and anti-angiogenic beads, are in various stages of research and development.46 Nanoparticles loaded with therapeutic agents, like PLGA nanoparticles with epigallocatechin gallate and doxycycline, have shown promise in preclinical studies for targeting and reducing endometriotic lesions.46 Surgical approaches, including robotic-assisted laparoscopy, are being refined to improvess outcomes for patients with severe endometriosis.28 Studies like the LAROSE trial aim to compare the efficacy and safety of these advanced surgical techniques with traditional methods.28
One promising area of research is the identification of non-invasive biomarkers for the diagnosis of endometriosis. Traditional diagnostic methods, such as laparoscopy, are invasive and not always feasible.10 Recent studies have identified distinct microbial communities in the peritoneal fluid and feces of endometriosis patients, suggesting potential biomarkers like Ruminococcus in the gut and Pseudomonas in the peritoneal fluid, which may aid in diagnosis with further investigation.15,18 Additionally, cytokine profiles in follicular fluid have shown significant differences between endometriosis patients and controls, with upregulation of IL-1β and IL-6 and lower levels of IL-12 and IL-10, providing another potential diagnostic avenue.47
Nanotechnology is an emerging field in the treatment of endometriosis.52 Nanoparticles can target endometriotic lesions for imaging and drug delivery, potentially improving the effectiveness of treatments while minimizing side effects.55,46 The summary for alternative drug delivery methods for endometriosis is represented by Table 6.52 For example, poly(lactic-co-glycolic) acid (PLGA) nanoparticles loaded with dual drugs (Figure 9A), such as epigallocatechin gallate and doxycycline, have shown promise in reducing oxidative stress, metalloproteases expression, and angiogenic factors in mouse models.46,47 Similarly, lipid nanoparticles have been demonstrated to target endometriotic tissues, suggesting their potential use in deep endometriosis.52,55
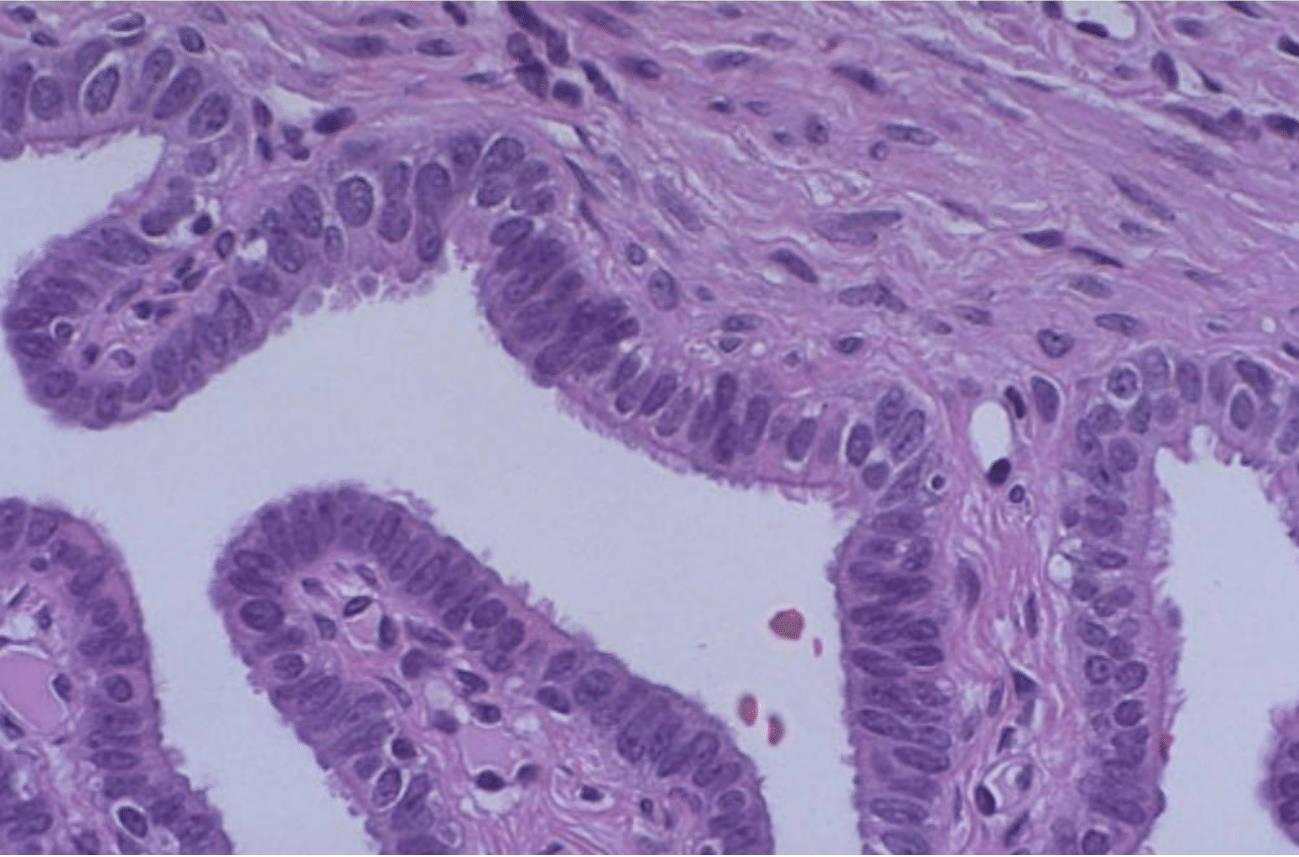
Figure 8 Healthy fallopian tube histology.62
The image shows a detailed histology of a healthy fallopian tube, serving as a baseline comparison for pathological conditions such as endometriosis.
|
Delivery method |
|
Drug |
Level of investigation |
Main results |
|
Vaginal ring |
Estroprogestins |
Clinical |
Conflicting results when compared with oral or transdermal routes. Limited investigation. |
|
|
Danazol |
Clinical |
Effective with lower serum level and AEs, not contraceptive. Ongoing investigation in patients with endometriosis |
||
|
Aromatase inhibitors |
Clinical |
Ongoing investigation in patients with endometriosis. |
||
|
Transdermal |
|
|
|
|
|
Intrauterine system |
Estroprogestins |
Clinical |
Compared only with vaginal ring. Limited investigation. |
|
|
Levonorgestrel |
Clinical |
Effective, higher or comparable satisfaction, contraceptive, not prevention of endometrioma |
||
|
Danazol |
Clinical |
Effective with lower serum level and AEs. Limited investigation |
||
|
Depot and long-acting formulations |
Injectable |
Depot Medroxyprogesterone acetate |
Clinical |
Effective, from higher to lower satisfaction based on comparison, irregular bleeding, concer om BMD loss. |
|
Implants |
Etonogestrel |
Clinical |
Effective, comparable to DMOA in terms of satisfaction, efficacy, and Aes |
|
|
GnRH analogs |
Clinical |
Multiple drug delivery option equally, 3 months depot formulation |
||
|
Nanotechnologies |
PLGA nanoparticles |
Epigallocatechin gallate + doxycycline |
Mouse model |
Antioxidant and antiangiogenic + inhibition of metalloproteases. Reduction of endometriotic implants |
|
Copaiba Oleoresin |
In vitro |
Antioxidant, anti-inflammatory, and antinociceptive. Reduced cell viability. |
||
|
Anti-CTLA-4 antibody |
Mouse model |
Inhibition of CD4+/CD25+/Tregs. Sustained release of antibodies. Inhibition of proliferation and invasion of endometriotic cells. |
||
|
anti-CRS antibodies |
Mouse model |
Reduce anti-inflammatory and pro-fibrotic activity of macrophages. Reduced proliferation and invasion ability of ectopic endometrial cells. |
||
|
AMNPs |
GMDP |
In vitro |
Increased stability and action of GMP. Enhanced expression of scavenger receptors and macrophages activity |
|
|
Lipid nanoparticles |
// |
Clinical |
Lipid nanoparticles are taken up by the endometriotic implants, adjacent healthy peritoneum, and surrounding endometrium. |
|
|
TNYL peptide |
HUaNS |
Mouse model |
Accumulation in and photothermal ablation of endometriotic implants without AEs |
|
|
nanoparticles |
silicon naphthalocyanine |
Rhesus macaques |
Accumulation in visualization of and photothermal ablation of endometriotic implants without Aes |
|
|
|
nanofibers |
curcumin |
Mouse model |
Continuous release of curcumin up to 30 days. Reduction of endometriotic implants and inflammatory infiltrate. |
Table 6 Alternative drug delivery methods for treatment of endometriosis52
PLGA, poly(lactic-co-glycolic) acid; AMNPs, aminopropyl mesoporous silica nanoparticles; GMDP, N-acetylglucosaminyl-N-acetamuramyl-L-alanyl-D-isoglutamine; HAuNS, holow goldnanospheres; AEs, adverse effects; BMD, bone mineral density.
Anti-angiogenic beads represent another innovative approach in treating endometriosis.68 These beads can inhibit the formation of new blood vessels necessary for the growth of endometriotic tissue.48 Research has shown that targeting angiogenesis can effectively reduce endometriotic lesion size and associated pain, although more clinical trials are needed to confirm these findings.18
Endometriosis is a widespread and debilitating condition affecting millions of women globally, significantly impacting their quality of life and reproductive health.1 The disease's pathophysiology involves the presence of endometrial-like tissue outside the uterus, leading to chronic inflammation, pain, and infertility.1–4,10 Despite advances in understanding its etiology—including theories of retrograde menstruation, coelomic metaplasia, genetic predisposition, hormonal dysregulation, and immune dysfunction—effective management remains challenging.10
The current treatment landscape includes pharmacological therapies such as NSAIDs, hormonal treatments (GnRH agonists, antagonists, progestins, and aromatase inhibitors), and surgical interventions like laparoscopy, laparotomy, and hysterectomy.30–41 Each treatment has its advantages and limitations, often focusing on symptom management rather than a cure. The diagnostic methods primarily involve laparoscopy, with emerging non-invasive techniques showing promise.38
Market analysis indicates substantial growth in the endometriosis treatment sector, driven by increasing disease prevalence, advancements in treatment options, and heightened awareness.7,29 Major contributors include pharmaceutical companies developing novel therapies, as well as surgical and diagnostic advancements.7,29 Despite these strides, the need for more effective, personalized treatments remains critical.10 The ongoing research and development efforts, particularly in non-invasive diagnostics and innovative therapies, hold promise for improving the quality of life for those affected by this condition.7,29
Eva Shlyakhovaya expresses appreciation to Professor Bill Tawil for overseeing the framework of this review, and for the insightful lectures and advice concerning tissue engineering, which contributed to the development of this paper.
Authors declare that there is no conflict of interest.
None.

©2024 Eva, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.