Journal of
eISSN: 2572-8466


Research Article Volume 1 Issue 3
Department of Botany and Microbiology, Al-Azhar University, Egypt
Correspondence: Saad El-Din Hassan, Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo-11884, Egypt
Received: December 08, 2016 | Published: December 28, 2016
Citation: Abdel-Rahman MA, Hassan SED, Azab MS, et al. Effective production of lactic acid by a newly isolated alkaliphilic Psychrobacter maritimus BoMAir 5 strain. J Appl Biotechnol Bioeng. 2016;1(3):68-76 DOI: 10.15406/jabb.2016.01.00012
The contamination risk during lactic acid production is one of the challenges to be overcome for effective fermentation because most reported lactic acid bacteria are neutrophilic strains. Therefore, in this study, a newly isolated alkaliphilic lactic acid bacterium was selected among several isolates obtained from natural sources. This isolate was identified as Psychrobacter maritimus BoMAir 5 using morphological, biochemical fermentation tests and16S rRNA gene sequencing. The strain exhibited homo lactic acid fermentation from glucose at pH 9.0. It also showed broad sugars utilization especially those derived from lignocellulosic biomasses. Cultural and nutritional conditions were optimized for efficient lactic acid fermentation. Lactic acid concentration of 140.8g/l with yield of 0.94g/g of consumed glucose was obtained using multi-pulse fed batch fermentation system with pH controlled at 9.0 and 40°C. For biotechnological application, Psychrobacter maritimus BoMAir 5 represents a potential strain for high lactic acid production under conditions that limit the contamination risk of fermentation.
Keywords: lactic acid production, alkaliphilic bacteria, fed-batch fermentation, psychrobacter maritimus
Lactic acid (LA) is very important chemical that is used for food, pharmaceutical, textile, cosmetic and chemical industries.1,2,3 LA is also an important building block in solvents and substances of biological activities4 and in the production of poly lactic acid, which is a biodegradable and biocompatible plastic material. Interestingly, pure L or D-LA can be only produced by microbial fermentation whereas chemical synthesis of LA produces a racemic mixture.5 Microbial fermentation of LA can be mainly achieved by some types of microorganisms e.g. fungi and LA producing bacteria.6 On the other hand, most of these organisms preferred the neutral or slightly acidic pH range of 5.5-6.57 that might increase the risk of contamination. Fermentation at alkaline conditions is one of the potential solutions to overcome this challenge, as these conditions are not favourable for most contaminant strains.8
Alkaliphilic microorganisms are defined as those preferred pH 9.0 or above for their optimal growth.9 These microorganisms can tolerate high level of salts especially mono valent ions as sodium ions that is an advantageous character.7 Their tolerance to these levels of salt and high value of pH could also minimize the risk of contamination.10 Alkaliphilic strains can also considered good producers for organic acids.11 Very few alkaliphilic strains have been reported for LA production from glucose such as Halolactibacillus halophilus that produced 65.8g/l L-LA in batch fermentation at pH 9.0 with a low yield of 0.76g/g,10 Exiguobacterium 8-11-1 strain produced 125g/l of L-LA with a yield of 0.98g/g in Fed batch fermentation at pH 8.5.12 Enterococcus casseliflavus 79w3 that produced 103g/l of L-LA with a low yield of 0.80g/g at pH 8.0 in batch fermentation13 and genetically modified Bacillus N16-5 that produced 143.9g/l of D-LA with a yield of 96.1g/g of glucose consumed in Fed batch fermentation.14 These studies either reported a low concentration of LA with low yield or performed at slightly alkaline conditions that increase the risk of contamination, in addition to the expected instability of genetically modified organisms. Therefore, this study aims to isolate wild-type alkaliphilic LAB from natural sources; in addition, screening and characterizing the most potent LA producer strain was evaluated. Moreover, optimization of the fermentation conditions for obtaining high LA production titre using different fermentation modes were determined.
Isolation and screening of alkaliphilic LAB
Forty soil and water samples collected from Alex, Matrouh and Wady EL-Natron and thirty-five samples of different dairy products were collected from different locations in Egypt. One gram of each solid sample or one ml of each liquid were suspended in 100ml of 0.85% NaCl, then 5 ml of the suspensions were added to 100mL-Erlenmeyer flask containing 50 ml of De Man, Rogosa and Sharpe (MRS) media that contained (g/l) glucose, 20; yeast extract, 5; poly peptone, 10; beef extract, 10; K2HPO4, 2; MgSO4, 0.1; MnSO4, 0.05; sodium acetate, 5; ammonium citrate, 2 and tween 80, 1ml and incubated at 37°C for 30h. Bacterial colonies were purified until obtaining single colonies. The obtained strains were grown on MRS agar medium supplemented with 0.4g/l bromocresol green to indicate acid production. All bacterial isolates were maintained in MRS media contained 15% glycerol at -80°C for storage. The obtained isolates were subjected to primary screening methods for selection the bacterial isolates by growing in MRS broth medium at 37°C for 30h. The selected isolates were then subjected to secondary screening in order to test the ability of bacteria to grow on high concentrations of glucose (50 and 100g/l) and sodium acetate (10 and 20g/l).
Characterization and identification of the most potent bacterial strain
Morphological (cell shape, arrangement, colony shape and colour), biochemical and physiological characteristics15,16 were determined using API 50 CHL test kit (bioMerieux, Marcy l’ ´Etoile, ´France). For the 16S rRNA analysis, genomic DNA was extracted according to modified method.17 An aliquot of DNA (1μl) was added to a polymerase chain reaction (PCR) reagent mix. Where, 16S rRNA was amplified in PCR using the genomic DNA as template and bacterial universal primers, 27f (5-GAGTTTGATCACTGGCTCAG-3) and 1492r (5-TACGGCTACCTTGTTACGACTT-3)18 to amplify an approximately 1.5Kb of 16S rRNA gene. The PCR mixture (50μl) contained 1×PCR buffer, 0.5mm MgCl2, 2.5 U Taq DNA polymerase (QIAGEN), 0.25mm dNTP, 0.5μM of each primer and 1μl of extracted bacterial genomic DNA. The PCR was performed in a DNA Engine Thermal Cycler (PTC-200, BIO-RAD, USA) with a hot starting performed at 94°C for 3min, followed by 30 cycles of 94°C for 0.5min, 55°C for 0.5min and72°C for 1min, followed by a final extension performed at 72°C for 10min. The PCR products were commercially sequenced by Sigma Company using ABI 3730xl DNA sequencer with the two primers. The 16S rRNA sequence was compared against the GenBank database using the NCBI BLAST program. Sequences were then compared with 16S rRNA sequences in the GenBank database using BLASTN. Multiple sequence alignment was done using ClustalX 1.8 software package and a phylogenetic tree was constructed by the neighbour joining method using MEGA (Version 6.1) software. The confidence level of each branch (1,000 repeats) was tested by bootstrap analysis.
Effect of different pH on LA fermentation
To study the effects of pH on LA production, batch fermentations were conducted at 37°C in the 100mL-Erlenmeyer flasks containing 50ml of MRS medium containing 20g/l glucose. The pH was adjusted at 5.0, 6.0, 7.0, 8.0, 8.5, 9.0, 10.0 and 11.0 where the control of pH was conducted using 10M NaOH. Samples (3ml of fermentation media) were taken every 3h of fermentation in the first 12 h and every 6 h after that until the end of fermentation period to measure cell growth (OD600), glucose consumption and LA concentration.
Effect of nitrogen sources on LA fermentation
In order to determine the best concentration of nitrogen sources for LA production, different concentrations of YE (0-10g/l), peptone (0-25g/l) and beef extract (0-25g/l) were performed individually in 100 mL-Erlenmeyer flasks containing 50ml of the media described above at 37°C for 30h. To study the optimal nitrogen source for bacterial growth and LA fermentation parameters by BoMAir 5, nitrogen sources of the MRS media were replaced by equivalent amounts of different organic and inorganic nitrogen sources. Soybean, urea, ammonium nitrate, ammonium sulphate, ammonium oxalate, ammonium chloride and ammonium ferric citrate were used individually as nitrogen sources in the 100 mL-Erlenmeyer flasks at the previous optimized conditions.
Optimization of temperature
The optimal temperature for LA fermentation by BoMAir 5 strain was tested in 100ml Erlenmeyer flasks containing 50ml medium as described above. An initial pH was adjusted to 9.0 by adding sterilized 10M NaOH. Fermentations were conducted statically at 25, 30, 37, 40, 45, 50, 55 and 60°C. Intermittent samples were taken along the fermentation period to determine the optimal temperature which used in the next step of optimization.
Effect of different carbon sources on LA fermentation
Different carbon sources (glucose, fructose, lactose, sucrose, raffinose, maltose, starch and cellulose) were also used to determine the optimal carbon source for LA fermentation. This experiment was achieved in MRS medium supplemented with different carbon sources at an initial concentration of 20g/l at 40°C for 30h and previous optimized conditions were considered. Moreover, different glucose concentrations of 20, 40, 60, 80, 100 and 150g/l were used to determine the glucose tolerance of strain BoMAir 5 at the previous optimized conditions. This experiment was performed under pH control at 9.0 using 10M NaOH.
Fed batch fermentation
Fed-batch fermentation was performed in a 1.0-l bioreactor with a working volume of 300ml of optimized medium. The pre-culture was as that used in the batch experiments. Fed-batch fermentation was initiated by the adding of 10% (v/v) inoculum. Temperature maintained at 40°C and the pH was controlled at 9.0 by NaOH (10M). Samples were taken every 3h at the first 108 h and every 6h after that and the culture growth (OD600), glucose concentrations and LA concentrations were determined. Feeding strategies were used for improving the fermentation efficiency. When the residual glucose concentration reached to 10g/l, glucose (30g/l) and YE (1.0g/l) were added to the bioreactor, these supplementations were repeated four times.
Analytical methods
The culture growth was estimated based on OD600 measurements using a visible spectrophotometer. After OD600 measurements, the cultures were centrifuged at 6,000 rpm for 10 min and the supernatants were analysed for glucose and LA determination. Residual glucose in the broth was estimated by using 3, 5-dinitrosalicylic acid reagent (DNS method).17 LA was measured by Barker and Summerson method.19 Firstly, LA was converted to acetaldehyde through oxidation with concentrated sulphuric acid. Acetaldehyde after that was coupled with p-hydroxy diphenyl in the presence of cupric ions forming a purple compound. The absorbance of this purple compound was measured by using spectrophotometer at 570nm. All experiments were conducted at triplicates, data were statistically analyzed by SPSS v17, analysis of variance (ANOVA) test was used for multiple sample comparison, when normality and homogeneity of variance were satisfied, followed by multiple comparison Tukey test.
Isolation and screening of lactic acid producing bacteria
In the current study, 170 alkaliphilic strains were isolated from different sources, where preliminary screening resulted in the selection of 34 isolates that detected to produce LA concentration more than 4.0g/l with high yield (>0.80g/g) of glucose consumed). Secondary screening tests were performed to select the most potent bacterial isolates based on their tolerance to high glucose concentrations (50 and 100g/l) and high sodium acetate concentrations (10 and 20g/l). Bacterial strain of BoMAir 5 was selected as the most potent among all rod shape and catalase positive bacterial isolates. This isolate appears to be homo fermentative LA bacterium because it can produce 9.6g/l of LA from 11.0g/l glucose with a yield 0.87g/g of glucose consumed.
Characterization and identification of the most potent isolate
Strain BoMAir 5 was identified based on morphological, physiological characters as shown in (Table 1). BoMAir 5 strain showed circular and convex colonies with white colour on agar plates. Cells are Gram negative, short rods shape, catalase positive. This strain can produce acid from several carbohydrates as shown in (Table 1). BoMAir 5 strain can grow at wide pH range of 7.0-10.0; however, the optimal pH for the maximum growth was 9.0, but it cannot grow at pH 5.0, 6.0 and 11.0. This strain cannot hydrolyse urea, pectin or cellulose, gelatine and citrate. Most properties are consistent with previous reported Psychrobacter strains.20,21 In contrast, strain BoMAir 5 differ from all other Psychrobacter strains in that it can utilize fructose as a sole carbon source producing LA homo fermentatively. Strain BoMAir 5 cannot tolerate higher concentration of NaCl than 7.5%. The acid production was compared to those of other Psychrobacter references strains such as Psychrobacter arenosus KMM 3659T20 and Psychrobacter piscatorii T-3-2T.21 Surprisingly, BoMAir 5 can tolerate higher temperature until 55°C with optimum temperature at 40°C that differ from other reported Psychrobacter strains that cannot grow above 37°C. In addition, Strain BoMAir 5 cannot haemolyse human blood agar plates indicating the bio-safety of this strain. Molecular identification based on 16s rRNA gene sequence analysis of BoMAir 5 strain showed the identity of 99% to Psychrobacter maritimus strain pi2-20 (accession number NR 027225). The phylogenetic tree showed that the topology of BoMAir 5 strain to Gamma proteo-bacteria (Figure 1). From these analyses, strain BoMAir 5 was identified as Psychrobacter maritimus BoMAir 5.
Morphological and biochemical characteristics |
Sugar fermentation |
||||
Character |
Sugar |
Recorded results |
Sugar |
Recorded results |
|
Cell shape |
Short rods |
Arbutin |
+ |
Starch |
+ |
Colony Color |
White |
Glycerol |
+ |
Glycogen |
|
Colony shape |
Convex |
Erythritol |
- |
Xylitol |
- |
Gram stain |
- |
D- Arabinose |
- |
Gentobiose |
- |
Catalase activity |
+ |
L-Arabinose |
- |
D-Turnose |
- |
Fermentation type |
Homo |
Ribose |
+ |
D-Lyxose |
- |
Growth temperature |
D-Xylose |
- |
D-Tagatose |
- |
|
25°C-55°C |
+ |
L-Xylose |
- |
D-Fructose |
- |
60°C |
- |
Adonytol |
- |
L-Fructose |
- |
Growth pH |
β-Methyle-d-xyloside |
- |
D-Arabitol |
- |
|
5.0-6.0 |
- |
Galactose |
- |
L-Arabitol |
- |
7.0-10.0 |
+ |
Glucose |
+ |
Gluconate |
+ |
11 |
- |
Fructose |
+ |
2-Keto-Gluconate |
- |
Tolerance to NaCl% |
Mannose |
- |
5-Keto-Gluconate |
- |
|
1.5-5.0 |
+ |
Sorbose |
- |
Esculin |
+ |
7.5-10.0 |
- |
Rhamnose |
- |
Salicin |
+ |
Hydrolysis of |
Dulcitol |
- |
Cellobiose |
+ |
|
Urea |
- |
Inositol |
- |
Maltose |
+ |
Citrate |
- |
Mannitol |
- |
Lactose |
- |
Pectin |
- |
Sorbitol |
- |
Melibiose |
- |
Starch |
+ |
α-Methyle-D-Mannoside |
- |
Sucrose |
- |
Cellulose |
- |
α-Methyl D-Glucoside |
- |
Trehalose |
+ |
Gelatin |
- |
N-Acetyle-Glucosamine |
+ |
Inulin |
- |
Blood |
- |
Amygdalin |
+ |
Melezitose |
- |
Raffinose |
- |
||||
Table 1 Morphological and biochemical characterization of BoMAir 5 strain.
+: Positive reaction, -: Negative reaction.

Figure 1 Phylogenetic analysis of 16S rRNA sequences of the bacterial isolate with the sequences from NCBI. Symbol ■ refers to 16S rRNA gene fragments retrieved from this study. The analysis was conducted with MEGA 6 using neighbor-joining method.
Effect of pH on lactic acid fermentation
The initial pH of the fermentation medium considered a critical factor for microbial growth and the selection of LA producing bacteria based on initial pH considers useful parameter in biotechnology.22 In the current study, batch fermentations were conducted at various pH values of 5.0, 6.0, 7.0, 8.0, 8.5, 9.0, 10.0 and 11.0 to determine the best pH value for LA fermentation by strain BoMAir 5. The optimum results for OD600 of fermentation broth, LA concentration, LA yield, LA productivity and maximum LA productivity were obtained at pH 9.0, with values of 0.76, 10.4, 0.90g/g of glucose consumed, 0.35g/l/h and 1.6g/l/h, respectively (Table 2). On the other hand, lower results recorded at other pH values while the growth was completely inhibited at pH values of 5.0, 6.0 and 11.0; so, no LA production was detected at these pH values (Table 2). Therefore, the pH 9.0 was selected for further investigations. From these results, Psychrobacter maritimus BoMAir 5 appeared to be more advantageous as LA producer because it was preferred alkaliphilic conditions than the common LAB, which preferred neutral to acidic conditions.23,24 Alkaliphilic strains tolerance to high levels of salts is helpful to reduce the contamination risk and decrease the amount of required neutralizing agents.10
pH value |
OD600 nm1 |
Consumed glucose (g/l) |
LA conc. (g/l)2 |
YLA (g/g)3 |
PLA (g/l/h)4 |
Max.PLA (g/l/h)5 |
5 |
0.153±0.01d |
0.0±0.0d |
0.0±0.0d |
0.0d |
0.00d |
0.00d |
6 |
0.343±0.01c |
0.0±0.0d |
0.0±0.0d |
0.0d |
0.00d |
0.00d |
7 |
0.540±0.01b |
4.10±0.1c |
3.60±0.1c |
0.88b |
0.12c |
0.2 (3h)c |
8 |
0.740±0.01a |
9.90±0.1b |
8.80±0.1ab |
0.89ab |
0.29b |
0.8 (3h)b |
8.5 |
0.760±0.01a |
10.2±0.2ab |
9.0±0.1a |
0.88b |
0.3a |
1.2 (3h)a |
9 |
0.680±0.01a |
11.5±0.1a |
10.4±0.1a |
0.90a |
0.35a |
1.6 (3h)a |
10 |
0.532±0.01b |
5.40±0.4c |
4.20±0.1c |
0.78c |
0.14c |
0.12 (3h)c |
11 |
0.187±0.01d |
0.0±0.0d |
0.0±0.0d |
0.00d |
0.00d |
0.00d |
Table 2 Effect of different pH values on the bacterial growth, glucose consumption, LA concentration, LA yield, LA productivity and maximum LA productivity from glucose by Psychrobacter maritimus BoMAir 5.
1OD: maximum optical density, 2Maximum lactic acid concentration after 30 h, 3Lactic acid yield, 4Lactic acid productivity at the end of fermentation time, 5Maximum lactic acid productivity at indicated time. Different letters between columns denote that mean values are significantly different (p≤0.05) by Tukey LSD test, means ± SE (n=3).
Optimization of fermentation medium
LA bacteria are fastidious organisms, which need complex nutrients as amino acids and vitamins for cell growth.25 Therefore, it is necessary to determine the optimal medial composition for the maximum LA production under limited nutritional conditions. Different concentrations of yeast extract (0-10g/l), peptone (0-25g/l) and beef extract (0-25g/l) were used individually as nitrogen sources in batch fermentations with initial pH 9.0. Cell growth, glucose consumption, LA concentration and LA productivity were gradually increased with increasing nitrogen sources concentration up to the optimal values. The optimal concentration of YE was 7.5g/l which gave values of 0.92, 11.7g/l, 10.6g/l and 0.35g/l/h, respectively. Also, the best concentration of peptone for all fermentation parameters by BoMAir 5 was 20g/l with values of 0.98, 12.0g/l, 10.8g/l and 0.36g/l/h, respectively. On the other hand, 10g/l beef extract appear to be the optimal concentration for LA fermentation by BoMAir 5 strain, where the recorded results were 0.82, 12.0g/l, 10.9g/l and 0.36g/l/h, respectively (Table 3). The highest LA concentration was reached to 10.9g/l after the optimization of nitrogen sources in the media.
Nitrogen Source (g/l) |
OD600 nm1 |
Consumed glucose (g/l) |
LA conc. (g/l)2 |
YLA (g/g)3 |
PLA (g/l/h)4 |
Max.PLA (g/l/h)5 |
|
Yeast extract conc. (g/l) |
0 |
0.88±0.007b |
6.7±0.2c |
6.0±0.2c |
0.90a |
0.20b |
0.80(3h)c |
2.5 |
0.89±0.005ab |
7.6±0.3c |
6.8±0.2c |
0.89a |
0.23b |
1.1(3h)b |
|
5 |
0.9±0.003a |
10.5±0.1a |
9.5±0.1ab |
0.89a |
0.31a |
1.3(3h)ab |
|
7.5 |
0.92±0.003a |
11.7±0.2a |
10.6±0.1a |
0.90a |
0.35a |
1.5(3h)a |
|
10 |
0.91±0.005a |
9.9±0.1b |
9.0±0.1b |
0.91a |
0.30a |
1.0(3h)b |
|
Peptone conc. (g/l) |
0 |
0.595±0.005d |
6.2 ± 0.1d |
5.6 ± 0.1d |
0.90b |
0.19d |
0.70 (3h)d |
5 |
0.695±0.001c |
7.4± 0.3c |
6.7±0.2c |
0.91ab |
0.22c |
0.80 (3h)c |
|
10 |
0.857±0.010b |
11.5± 0.1ab |
10.2±0.1a |
0.90b |
0.27c |
1.0 (3h)b |
|
15 |
0.872±0.004b |
11.6± 0.1ab |
10.3±0.2a |
0.92a |
0.31b |
1.2 (3h)b |
|
20 |
0.983±0.005a |
12.0± 0.1a |
10.8±0.1a |
0.90b |
0.36a |
1.6 (3h)a |
|
25 |
0.834±0.005b |
11.0 ±0.1b |
9.8±0.1b |
0.89c |
0.33a |
1.3 (3h)ab |
|
Beef extract |
0 |
0.752 ±0.002b |
5.5 ±0.1e |
4.9±0.1e |
0.89b |
0.16d |
0.8c |
5 |
0.761 ±0.002b |
8.1±0.4c |
7.3 ±0.3c |
0.90a |
0.24c |
1.2b |
|
10 |
0.822 ±0.007a |
12.2 ±0.1a |
10.9±0.1a |
0.89b |
0.36a |
1.6a |
|
15 |
0.803 ±0.005a |
10.1 ±0.2b |
9.1 ±0.2b |
0.90a |
0.30b |
1.3ab |
|
20 |
0.81 ±0.002a |
8.5±0.1c |
7.6±0.0c |
0.89b |
0.25c |
1.2b |
|
25 |
0.744±0.001b |
6.5±0.1d |
5.8±0.1d |
0.89b |
0.19d |
0.9c |
|
Table 3 Effect of different concentrations of yeast extract, peptone and beef extract on the bacterial growth, glucose consumption, LA concentration, LA yield, LA productivity and maximum LA productivity from glucose by Psychrobacter maritimus BoMAir 5.
1OD: maximum optical density; 2Maximum lactic acid concentration after 30 h, 3Lactic acid yield, 4Lactic acid productivity at the end of fermentation time, 5Maximum lactic acid productivity at indicated time. Different letters between columns denote that mean values are significantly different (p≤0.05) by Tukey LSD test, means ±SE (n=3).
Higher concentrations of nitrogen sources led to decrease of all fermentation parameters indicating that these high concentrations might to be toxic as previously reported.26 In comparison with other reports, several researchers used YE as the sole organic nitrogen source.27,28,29,30 In order to substitute nitrogen sources in above optimized MRS media (7.5g/l YE, 20g/l peptone and 10g/l beef extract) with another inexpensive nutrient, seven nitrogen sources were used to evaluate their effects on LA production by Psychrobacter maritimus BoMAir 5. Unfortunately, none of these sources gave higher growth, sugar consumption, LA concentrations and LA productivity as obtained using organic sources in media by selected strains in this study (Table 4). The importance of yeast extract, peptone and beef extract supplement could be explained by the fact that it contains critical amounts of vitamins and trace elements essential for LA biosynthesis.26 In the other studies, the peanut meal concentration of 20g/l was the most favourable for L-LA production by Bacillus coagulans WCP10-4.7
Nitrogen source |
OD600nm1 |
Consumed glucose (g/l) |
LA conc. (g/l)2 |
YLA (g/g)3 |
PLA (g/l/h)4 |
Max.PLA (g/l/h)5 |
Soybean |
0.630±0.003b |
8.0±0.2b |
7.4±0.1b |
0.93a |
0.25b |
1.5 (3h)b |
Urea |
0.157±0 .01d |
2.6±0.1c |
2.3±0.1c |
0.88b |
0.08c |
0.5 (3h)c |
Amm. Nitrate |
0.153±0.004c |
2.4±0.0c |
2.0 ±0.1d |
0.83b |
0.07c |
0.6 (3h)c |
Amm. Sulphate |
0.191±0.002c |
2.4 ± .1c |
1.9±0.1d |
0.79c |
0.06c |
0.6 (3h)c |
Amm. Oxalate |
0.196± 0.005c |
2.7±0.0c |
2.3±0.0c |
0.85b |
0.08c |
0.6 (3h)c |
Amm. Chloride |
0.177± 0.004c |
2.2±0.1d |
1.8±0.1d |
0.82b |
0.06c |
0.5 (3h)c |
Amm ferric citrate |
0.110± 0.01dd |
0e |
0e |
0d |
0d |
0d |
Control |
0.994±0.01a |
11.6±0.0a |
10.5±0.2a |
0.91a |
0.35a |
1.8 (3h)a |
Table 4 Effect of different nitrogen sources on the bacterial growth, glucose consumption, LA concentration, LA yield, LA productivity and maximum LA productivity from glucose by Psychrobacter maritimus BoMAir 5.
1OD, maximum optical density; 2Maximum lactic acid concentration after 30 h, 3Lactic acid yield, 4Lactic acid productivity at the end of fermentation time, 5Maximum lactic acid productivity at indicated time. Different letters between columns denote that mean values are significantly different (p≤0.05) by Tukey LSD test, means ± SE (n=3).
Effect of temperature
Determination the optimal temperature degree for bacterial growth significantly affect the rate of biochemical reactions, generation time, bacterial enzymatic activity as well as conversion rate of different substrates.31 Table 5 summarized fermentation parameters for LA production by BoMAir 5 at different temperatures (25-60°C) in batch fermentation mode. It was observed that 40°C represented the optimal temperature for LA fermentation parameters by BoMAir 5 where the maximum values of OD600 (0.86), glucose consumption (12.3g/l), LA concentration (11.2g/l) and LA productivity (0.37g/l/h) were obtained (Table 5). However, increasing or decreasing in the temperature degree than that the optimal value, the microbial activity was substantially reduced due to most of bacterial enzymes were probably denatured. The final LA concentration was increased to 11.2g/l than that obtained in the previous experiment (10.9g/l). Surprisingly, this strain is thermotolerant; that preferred 40°C as an optimal fermentation temperature which is also an advantageous character to reduce the possibility of contamination risk. This thermotolerant characteristic is in contrary to Psychrobacter maritimus sp. nov that could grow only at 4-37°C, with an optimal growth temperature of 25-28°C but does not grow at 39-40°C.20
Temperature(°C) |
OD600nm1 |
Consumed glucose (g/l) |
LA conc. (g/l)2 |
YLA (g/g)3 |
PLA (g/l/h)4 |
Max.PLA (g/l/h)5 |
25 |
0.590±0.002c |
7.10± 0.1d |
6.50±0.1d |
0.92a |
0.22b |
0.80 (3h)b |
30 |
0.630±0.005b |
9.30 ±0.1 |
8.40±0.1c |
0.90a |
0.28b |
1.0(3h)a |
35 |
0.640±0.010b |
10.6 ±0.2b |
9.60±0.1b |
0.91a |
0.32a |
1.0(3h)a |
40 |
0.860±0.004a |
12.3±0.1a |
11.2±0.1a |
0.91a |
0.37a |
1.3(3h)a |
45 |
0.630±0.002b |
11.1±0.2a |
10.0±0.1a |
0.90a |
0.33a |
1.0(3h)a |
50 |
0.520±0.007d |
9.00 ±0.1c |
8.10±0.1c |
0.90a |
0.27b |
1.0(3h)a |
55 |
0.430±0.07e |
8.10±0.1c |
7.30±0.1d |
0.90a |
0.24b |
0.90(3h)b |
60 |
0.210±0.006f |
0.00e |
0.00e |
0.0b |
0.00c |
0.0c |
Table 5 Effect of different temperatures on the bacterial growth, glucose consumption, LA concentration, LA yield, LA productivity and maximum LA productivity from glucose by Psychrobacter maritimus BoMAir 5.
1OD, maximum optical density; 2Maximum lactic acid concentration after 30 h, 3Lactic acid yield, 4Lactic acid productivity at the end of fermentation time, 5Maximum lactic acid productivity at indicated time. Different letters between columns denote that mean values are significantly different (p≤0.05) by Tukey LSD test, means ± SE (n=3).
Optimization of carbon source
For industrial process, it is economically useful that the selected LA producing-bacteria have the ability to metabolize the different carbohydrates into optically pure LA through homo fermentative pathway without by-product formation.32 The presented study showed that, from the different carbon sources that can utilized by strain BoMAir 5, glucose and fructose caused the highest LA production of 10.9 g/l (Table 6), while the yield was higher in the case of glucose.
Carbon source |
OD600 nm1 |
Consumed sugar(g/l) |
LA conc. (g/l)2 |
YLA (g/g)3 |
PLA (g/l/h)4 |
Max PLA (g/l/h)5 |
Glucose |
0.723±0.006a |
12.0±0.1a |
10.9±0.1a |
0.91a |
0.36a |
1.3 (3h)a |
Fructose |
0.76±0.011a |
12.6±0.1a |
10.9±0.1a |
0.86a |
0.36a |
1.1 (3h)a |
Lactose |
0.545±0.013c |
0.0±0.0c |
0.0±0.0c |
0.0b |
0.0b |
0.0b |
Sucrose |
0.495±0.007d |
0.0±0.0c |
0.0±0.0c |
0.0c |
0.0c |
0.0b |
Raffinose |
0.437±0.003d |
0.0±0.0c |
0.0±0.0c |
0.0d |
0.0c |
0.0c |
Maltose |
0.695±0.003b |
2.3±0.0b |
1.9±0.1b |
0.83b |
0.06b |
0.19 (3h)b |
Starch |
0.489±0.009d |
0±0.0c |
0.0±0.0c |
0.0d |
0.0c |
0.0c |
Cellulose |
0.504±0.008c |
0±0.0c |
0.0±0.0c |
0.0d |
0.0c |
0.0c |
Table 6 Lactic acid production using different carbon sources by Psychrobacter maritimusBoMAir 5.
1OD, maximum optical density; 2Maximum lactic acid concentration after 30 h, 3Lactic acid yield, 4Lactic acid productivity at the end of fermentation time, 5Maximum lactic acid productivity at indicated time. Different letters between columns denote that mean values are significantly different (p≤0.05) by Tukey LSD test, means ± SE (n=3).
Effect of different glucose concentration
The high initial substrate tolerance is very important for getting high LA production to reduce the downstream processing costs.33 In batch fermentation, BoMAir 5 strain exhibited the growth increase with the increase of glucose concentrations up to 60g/l recording the maximal OD 3.93, while the growth decreased after that. The results represent in (Figure 2) indicated substrate inhibition that resulted in long lag phase at higher concentrations above 60g/l followed by short log phase and very long stationary phase. Final LA concentration was increased from 18.9g/l up to 67.9g/l using initial glucose concentrations of 20g/l and 150g/l, respectively. Therefore, the residual glucose concentration was very high (80g/l) when using 150g/l glucose. Surprisingly, BoMAir 5 strain metabolizes glucose homo fermentatively at all tested concentrations, where yielding LA range of 0.95-0.97 g/g of glucose consumed. On the other hand, LA productivity was increased with the increase of initial glucose concentration up to 40 g/l while become decreased vigorously when the initial glucose concentration was higher than 40g/l. Therefore, the 40g/l glucose was selected as the optimal concentration for further studies.
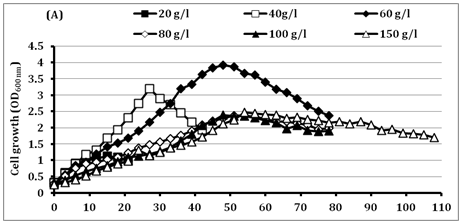
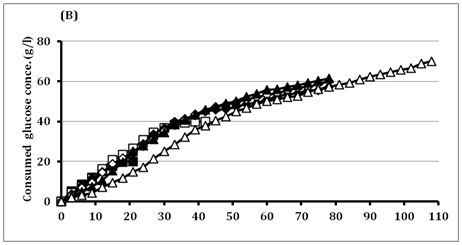
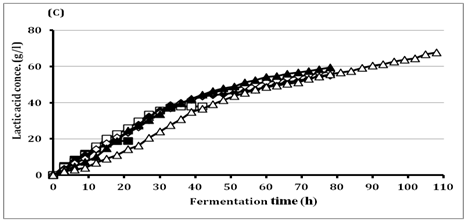
Figure 2 Effect of different glucose concentrations on
(A) Cell growth
(B) Glucose consumption and
(c) Lactic acid production by BoMAir 5 at 40°C.
Symbols: ■ 20 g/l; □ 40 g/l; ♦ 60 g/l; ◊ 80 g/l; ▲ 100 g/l; Δ 150 g/l.
Fed batch fermentation
The batch culture in most cases produces lower LA concentration, biomass and productivity than other fermentation modes. This could have resulted from the high osmotic pressure on microbial cells in the batch culture condition and the reduced water activity combined with plasmolysis caused by high substrate concentration that results in a decrease in the fermentation rate and sugar consumption.26 The current study assumed that, using low initial glucose concentration at the beginning of fermentation and feeding with sugars during fermentation, the effects of glucose inhibition on LA production would be avoided and the fermentation efficiency would be greatly enhanced. Therefore, we conducted Fed batch fermentations with multi-pulse feeding under pH control at pH 9.0 using 10M NaOH as a neutralizing agent. The initial glucose concentration was 40g/l and the multi-pulse (four) feedings were achieved by 30g/l of glucose and 1g/l of YE when the glucose concentration reached to 10.0g/l (Figure 3).
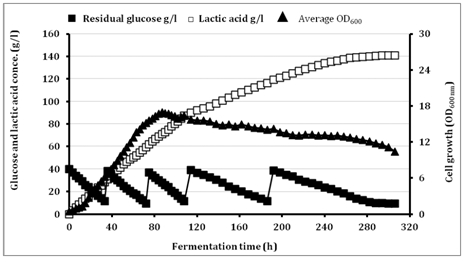
Figure 3 Lactic acid production in fed batch fermentation by BoMAir 5 using initial glucose concentration of 40g/l at 40°C. Symbols: ■ residual glucose; □ average LA, ▲ Average optical density.
The growth of BoMAir 5 strain was increased with time up to 81 h where they obtained OD600 was 16.3 and become stable for another 27h then decreased gradually until fermentation was ended recording OD600 of 10.44 at 306h. Final LA concentration was improved with 107.3% than at batch fermentation where 140.8g/l LA with low residual glucose concentration of 9.48g/l were achieved with Fed batch fermentation than in case of batch method where only 67.9g/l of LA with very high residual glucose of 80g/L using 160g/l and 150g/L glucose, respectively. Interestingly, LA yield at all fermentation time were ranged 0.93-0.99g/g of glucose consumed indicating that, BoMAir 5 strain metabolized glucose homo fermentatively where only 2.6g/l acetic acid was detected at the end of fermentation. On the other hand, LA productivity gave the highest value at the early stage of fermentation while decreased at other stages as a result of accumulation of LA concentration causing end product inhibition. Except for an alkalophilic Bacillus sp. WL-S20 that produced 225g/l from glucose at a yield of 99.3g/g using multi-pulse Fed batch fermentation,7 the final LA concentration (140.8g/l) and LA yield (0.93-0.99g/g of glucose consumed) obtained in the current study under alkaliphilic condition using the green neutralizer (NaOH) to maintain the pH during fermentation were acceptable when compared with other wilD-type alkaliphilic strains e.g Halolactibacillus halophilus (65.8g/l L-LA with yield of 0.76g/g),10 Exiguobacterium 8-11-1 (125g/l of L-LA with a yield of 0.98g/g),12 Enterococcus casseliflavus 79w3 (103g/l of L-LA with yield of 0.80g/g).13
In this study, a novel alkaliphilic bacterial strain of BoMAir 5 was isolated and identified as Psychrobacter maritimus BoMAir 5. This strain showed the highest capacity to produce LA under alkaline conditions using different glucose concentrations. Fermentation conditions were optimized in batch fermentations, where efficient production of polymer-grade lactate by alkaliphilic bacterial strain of BoMAir 5 was established using Fed batch fermentation technique. Psychrobacter maritimus BoMAir 5 has merits of high pH adaptation (9.0), high yield of glucose to LA (0.93-0.99g/g of glucose consumed) and high LA production titre (140.8g/l). Alkaliphilic characteristic of BoMAir 5 strain can be useful to reduce the risk of contamination during fermentation technique. Considering the above performance, Psychrobacter maritimus BoMAir 5 appears to be suitable for industrial scale production of lactic acid.
None.
The author declares no conflict of interest.

©2016 Abdel-Rahman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.