Journal of
eISSN: 2572-8466


Research Article Volume 7 Issue 4
1Department of Biology, University of Córdoba, Colombia
2Department of Biology, Pontificia Universidad Javeriana, Colombia
Correspondence: Jesús Ballesteros Correa Unicórdoba Biodiversity Research Group, Department of Biology, University of Córdoba, Colombia
Received: May 11, 2020 | Published: July 6, 2020
Citation: Ballesteros J, Perez-Torres J. Effect of extensive livestock management on the diversity of bats (Chiroptera) in Cordoba, Colombia.J Appl Biotechnol Bioeng. 2020;7(4):149-155. DOI: 10.15406/jabb.2020.07.00227
Extensive livestock farming has caused negative effects on biodiversity, affecting the functioning of dry ecosystems in the Colombian Caribbean. The objective was to evaluate the eeffect of livestock management on the diversity of bats associated with bs-T fragments. During a year-long fieldwork, I know They identified 39 species of bats distributed in 23 genera and six families. The Phyllostomidae family presented the highest species richness, with Stenodermatinae being the most abundant group. The assembly of species in the SSP presented greater equity, with a relative abundance by species and foraging guilds, significantly higher in the SSP. The capture success presented a significant temporal variation (rains and drought), with greater abundance during the rainy season. Artibeus planirostris, Artibeus lituratus, Carollia perspicillata, Carollia castanea, Phyllostomus discolor, Dermanura phaeotis, Uroderma convexum, Glossophaga soricina, C. brevicauda were the most abundant species. Fruit bats showed greater temporal stability in SSP environments; while, in SC fragments the rate of species turnover was higher. The research indicates a positive effect of silvopastoral management of extensive livestock farming on bat diversity, diminishing the negative effect of biodiversity loss.
Keywords: chiroptera, species diversity, conservation, silvopastoral system, Colombian caribbean
Chiropterans are highly diverse flying mammals that participate in fundamental ecological processes and dynamics of tropical forests,1,2 as well as in the provision of ecosystem service.3 These small flying mammals, being seed dispersers, especially of pioneer species and trees in secondary forests, are responsible for the natural regeneration of forests;4,5 they also play an important role in the control of insect populations3,6 and in the pollination of more than 800 species of neotropical plants.7
In the Colombian Caribbean region, large extensions of tropical dry forest (bs-T) have been transformed into paddocks of extensive livestock,8 with negative effects on biodiversity and ecosystem functions; and despite representing the only refuge for the biodiversity of this type of ecosystem, it only has less than 2% of its original coverage, and is represented by fragments immersed in livestock landscapes, with a high risk of extinction. In this scenario of landscape transformation, climate change and resource alteration, a situation that affects some species and favors others, many fauna groups change their structure and composition of species.9,10 Bats' tolerance of landscape changes and habitat alteration depends on their ability to cross open areas between forest fragments,11,12 a characteristic that enables access to a diversity of habitats that can provide food and shelter requirements.
Several investigations have provided information on the effects of landscape transformation and habitat modifications on bat assembly;13–15 but few studies evaluate the effect of extensive livestock management type on bat assembly in bs-T areas.It has been argued that SSP can counteract the loss of biodiversity caused by conventional extensive livestock (SC), and that it may be a viable strategy for the conservation of bs-T and its biodiversity. This work generates information on the effects of the implementation of the extensive livestock SSP on the assembly of bats in BS-T areas of the Colombian Caribbean.
Study area: In four extensive livestock farms with bs-T fragments in the department of Córdoba, Colombia. Two fragments associated with silvopastoral management systems (SSP) and two associated with conventional (SC) of extensive livestock were evaluated, a warm climate, temperature 28°C and 1300 mm of annual precipitation with unimodal distribution, rainy season (May- November) and dry season (December-April).Among the evaluated localities, two are associated with the SSP (Fincas Las Palmeras-Montería with 560 ha, and San Lorenzo-Los Córdobas with 860 ha); the other two fragments are associated with the SC (Fincas Chimborazo-Canalete with 470 ha, and Guacamayas-Buenavista with 450 ha).
Sampling and data collection: During From August 2011 to July 2012, 15 samplings were carried out over three consecutive nights, for a sampling effort of 7560 net-hours / night for each fragment. The bats were captured using mist nets (6x3 m), following the methodology.16,17 Fourteen fog nets were installed: five floor nets and five nets raised more than 4 m inside each fragment, and four floor nets on the outskirts of the fragments near the edge. The nets were deployed from 6:00 p.m. - 6:00 a.m. the following day, and reviewed every 45 minutes, for a total sampling effort of 30,240 net-hour /night.
The collected bats were processed in situ; With the 0.02 mm precision caliper, the standard morphometric measurements were taken (wingspan, body length, tail, ear, swallow, nasal blade, forearm, tibia, calcaneus), sex and weight with a 0.01 g precision electronic scale. For the identification of the species, specialized taxonomic keys were used,18–20 and descriptions of of Gardner.21 The taxonomy was carried out according to Wilson & Reeder22 trophic guilds of the species were determined according to Soriano.23,24
A reference collection was deposited in the Javeriano Museum of Natural History of the Pontificia Universidad Javeriana in Bogotá (MPUJ-MAMM), under the collection numbers MPUJ-MAMM: 1911-2186. During the study, the ethical, technical-scientific and administrative standards for animal research contained in Law 84 (National Congress of Colombia 1989) were taken into account, withCVS Montería research permit, Resolution No. 2-1033 (05-22-2015). 2.3. Information analysis. Relative abundance (AR) and capture success rate were calculated.25 Variance analysis was performed to purchase the EC by type of management (SSP and SC), species, genus and foraging union, after reviewing assumptions (normality: Shapiro-Wilk and Kolmogorov-Smirnov; homogeneity of variances: Barlett), transformations for normality adjustment and ex post tests (Tukey) when applicable. The averages of the capture success (EC), transformed for grouped species (EC-0.2), for genera (EC-0.25) and for grouped foraging guilds (EC-0.1). All statistical analyzes were carried out in the R computational statistics program. Dominance was calculated with the Simpson index and the Pielou Equity Index.26 The representativeness of the samplings was evaluated byspecies accumulation curves for each level of design factor, and adjusted to the nonparametric Chao1 and Bootstrap models, with a 95% CI, estimating the number of expected species.27 The analysis of the diversity of the species assembly was carried out using the concept of "true diversity"28,29 with iNEXT30,31 via the RStudio command editor in the R language. The degree of change in species composition between SSP and SC fragments was determined; and between the dry-rainy seasons in both management systems.32,33 The similarity in the composition of species between localities and management systems was determined using the Morisita similarity index,26 and the complementarity of species in the assembly of bats between the silvopastoral and conventional management systems was calculated.
The EC in the fragments in SSP (12.42 individuals / hour-mesh) was significantly higher than in the SC fragments, with 6.02 individuals / hour-mesh (p <0.001), and 67.4% of the total captures. The EC presented significant differences between fragments of the SSP and SC, in terms of grouped species (p <0.0001); of genders (p <0.0001); and of grouped foraging unions (p <0.001). In all three cases, the EC of bats was higher in the SSP, without significant differences between fragments of the same management system. In both management systems there was temporal variation in the success of catching EC of bats (Figure 1).
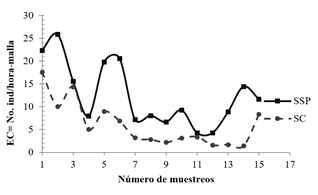
Figure 1 Successful capture of bats in bs-T fragments in the silvopastoral (SSP) and conventional (SC) system of extensive livestock farming in Córdoba, Colombia.
(EC)^(-0.2) (EC)^(-0.25) (EC)^(-0.1)
There were significant differences in the temporal variation (rainy season and dry season) in bat abundances (p <0.02), the EC being higher during the rainy season. The Phyllostomidae family presented the highest abundance and species richness, at both times of the year. Carollia perspicillata, C. castanea, Artibeus lituratus, Dermanura phaeotis, Uroderma convexum and Sturnira lilium, presented the highest abundance. There was temporal variation in abundance in foraging guildsin both management systems; and during the rainy season, all foraging guilds showed consistently higher relative abundance than in the dry season (Figure 2). Lfrugivores were 2.42 times more abundant inthe fragments of the SSP, which in the SC.
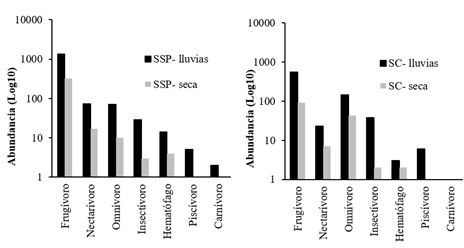
Figure 2 Temporal changes (rainfall-drought) in the relative abundance of foraging guilds in bs-T fragments in the SSP (left) and SC (right) of extensive livestock farming in Córdoba, Colombia.
In 2,788 captures made, 39 species of bats belonging to 23 genera and six families (Phyllostomidae, Emballonuridae, Vespertilionidae, Molossidae, Mormopidae and Noctilionidae) were identified. Phyllostomidae presented higher species richness (30); and of the subfamilies, Stenodermatinae was more diverse (12 species) and abundant (n = 1545); while Lonchophyllinae presented the lowest abundance (Table 1). Artibeus planirostris and A. lituratus were the most abundant species in both types of management, with 76.84% in the SSP and 61.87% in SC. Uroderma convexum, Dermanura phaeotis, Carollia perspicillata and C. castanea had high abundance only in the SPP; while in the SC only Phyllostomus discolor had high abundance (Table 1). 29 species presented low abundance (1-2 individuals), constituting 8% of the catches. for the fragments associated with the SSP, while five species were for the fragments of the SC.
|
Family/ Subfamily |
Species |
Guild |
SSP |
|
SC |
|
Total |
|
Palm |
SLor |
Chim |
Guac |
||||
|
Phyllostomidae |
|||||||
|
Stenodermatinae |
Artibeus planirostris |
F |
110 |
166 |
111 |
187 |
574 |
|
Artibeus lituratus |
F |
52 |
254 |
73 |
30 |
409 |
|
|
Artibeus jamaicensis |
F |
two |
one |
one |
0 |
4 |
|
|
Platyrrhinus helleri |
F |
4 |
10 |
two |
5 |
twenty-one |
|
|
Platyrrhinus angustirostris |
F |
0 |
0 |
two |
one |
3 |
|
|
Platyrrhinus umbratus |
F |
one |
0 |
0 |
0 |
one |
|
|
Uroderma convexum |
F |
66 |
70 |
one |
38 |
175 |
|
|
Uroderma magnirostrum |
F |
4 |
one |
0 |
one |
6 |
|
|
Vampyriscus nymphaea |
F |
two |
5 |
3 |
4 |
14 |
|
|
Sturnira lilium |
F |
56 |
25 |
one |
16 |
98 |
|
|
Dermanura phaeotis |
F |
4 |
187 |
8 |
2. 3 |
222 |
|
|
Dermanura cf watsoni |
F |
0 |
18 |
0 |
0 |
18 |
|
|
Carolliinae |
Carollia perspicillata |
F |
86 |
228 |
39 |
37 |
390 |
|
Carollia castanea |
F |
98 |
122 |
eleven |
14 |
245 |
|
|
Carollia brevicauda |
F |
28 |
49 |
12 |
19 |
108 |
|
|
Glossophaginae |
Glossophaga soricina |
N |
26 |
61 |
22 |
7 |
116 |
|
Glossophaga commissarisi |
N |
0 |
two |
one |
0 |
3 |
|
|
Lionycteris spurrelli |
N |
0 |
one |
0 |
0 |
one |
|
|
Lonchophyllinae |
Lonchophylla robusta |
N |
one |
0 |
0 |
0 |
one |
|
Hsunycteris thomasi |
N |
0 |
0 |
one |
0 |
one |
|
|
Phyllostominae |
Lophostoma silvicolum |
I |
two |
0 |
0 |
fifteen |
17 |
|
Lophostoma brasiliense |
I |
0 |
0 |
one |
one |
two |
|
|
Micronycteris megalotis |
I |
0 |
0 |
0 |
two |
two |
|
|
Micronycteris hirsuta |
I |
0 |
one |
0 |
0 |
one |
|
|
Phyllostomus discolor |
OR |
24 |
37 |
125 |
37 |
223 |
|
|
Phyllostomus hastatus |
OR |
6 |
13 |
twenty |
6 |
Four. Five |
|
|
Phyllostomus elongatus |
OR |
one |
0 |
0 |
0 |
one |
|
|
Gardnerycteris crenulatum |
I |
one |
0 |
two |
0 |
3 |
|
|
Trachops cirrhosus |
C |
two |
0 |
0 |
one |
3 |
|
|
Desmodontinae |
Desmodus rotundus |
H |
13 |
5 |
3 |
two |
2. 3 |
|
Emballonuridae |
|||||||
|
Emballonurinae |
Saccopteryx leptura |
I |
3 |
6 |
one |
3 |
13 |
|
Saccopteryx bilineata |
I |
one |
0 |
0 |
0 |
one |
|
|
Vespertilionidae |
|||||||
|
Myotis nigricans |
I |
one |
two |
0 |
0 |
3 |
|
|
Myotis nesopolus |
I |
0 |
0 |
one |
0 |
one |
|
|
Rhogeessa io |
I |
5 |
two |
0 |
two |
9 |
|
|
Molossidae |
|||||||
|
Molossus molossus |
I |
3 |
4 |
0 |
9 |
16 |
|
|
Molossops temminckii |
I |
0 |
0 |
0 |
two |
two |
|
|
Mormoopidae |
|||||||
|
Pteronotus davyi |
I |
one |
0 |
0 |
0 |
one |
|
|
Noctilionidae |
|||||||
|
Noctilio albiventris |
P |
one |
4 |
0 |
7 |
12 |
|
|
Total |
|
|
604 |
1274 |
441 |
469 |
2788 |
Table 1 Taxonomy and abundance of bats in fragments of bs-T associated with SSP (Palm: Palmeras and SLor: San Lorenzo) and SC (Chim: Chimborazo and Guac: Guacamayas) from livestock in Córdoba, Colombia
F, frugivore; N, nectarivore; I, insectivore; H, hematophagus; P, piscivore; O, omnivore; C, carnivore
Temporarily, there were significant differences in the EC of bats between the dry season and the rainy season (p <0.05); with higher CD during the rainy season in both management systems. No significant differences were found in the sex ratio between the management systems. Significant differences were found in the average weight of the species A. lituratus (p <0.001), U. convexum (p <0.003), and C. brevicauda (p <0.05), with a higher weight in bats of the fragments associated with the SSP.
Fruit bats were significantly more abundant in the SPP fragments (82%) than in SC throughout the sampling cycle (p <0.02); the Nectarivorous, insectivorous and omnivorous guilds did not show significant differences between the two types of management of the livestock system. There wasa highly significant association of the SSP with the frugivores by grouped species (p <0.001), and by grouped genera (p <0.01); and highly significant effects of the type of management were detected on the success of capture by foraging guilds between localities (p <0.0001). 0D and 1D true alpha diversity was higher in fragments of the SSP, with a level of completeness greater than 98% in all cases, compared to SC (Table 2). The bs-T fragments in SSP had 1.16 times more 1D bat diversity than in SC; that is, the SC fragments have 86% of the species that are in the SSP fragments; and on average, the SSP fragments presented 2.87 more species than in the SC fragments.
|
Locations |
N |
0D |
oneD |
twoD |
Completeness |
|
Driving / Epoch |
(Abundance) |
(Wealth sp.) |
Exp (entropy H ') |
(1 / Simpson) |
(%) |
|
The Palm trees |
604 |
29±4.14 |
11.51±1.00 |
8.76±0.72 |
98.7 |
|
San Lorenzo |
1274 |
25±2.90 |
10.12±0.51 |
7.82±0.42 |
99.7 |
|
Macaws |
469 |
25±2.72 |
9.76±1.06 |
5.25±0.80 |
99.2 |
|
Chimborazo |
441 |
22±4.32 |
7.55±0.86 |
5.40±0.58 |
98.2 |
|
Silvopastoral management |
1878 |
33±4.26 |
11.44±0.50 |
8.88±0.37 |
99.6 |
|
Conventional handling |
910 |
30±3.18 |
9.83±0.87 |
6.01±0.65 |
99.3 |
|
Rainy season |
2285 |
36±4.20 |
11.90±0.52 |
8.68±0.46 |
99.7 |
|
Dry season |
503 |
23±5.35 |
10.06±0.90 |
7.89±0.66 |
98.2 |
|
Rainy season-SSP |
1522 |
31±4.09 |
11.61±0.56 |
9.00±0.43 |
99.5 |
|
Rainy season-SC |
763 |
29±3.38 |
9.46±0.94 |
5.60±0.64 |
99.2 |
|
Dry season-SSP |
356 |
20±4.14 |
8.79±0.89 |
6.70±0.61 |
98.3 |
|
Dry season-SC |
147 |
15±2.85 |
9.35±1.23 |
7.61±1.12 |
97.3 |
Table 2 True alpha diversity of bats in bs-T fragments, with SSP and SC management of extensive livestock farming in Córdoba, Colombia. Three measures of diversity are presented: 0D, 1D and 2D with a confidence of 95%, and completeness of the sampling
True beta diversity or species turnover rate of bats between SSP and SC from extensive cattle ranching was 1.24 communities (61.9%) with q=0. Beta diversity of order q=1 was 1.11 effective communities (55.4%); while the beta diversity of order q=2, which only includes typical species, was 1.16 effective communities (58.2%). Temporarily, the replacement of species (0D) between dry and rainy season was higher in the SC fragments with 1.36 effective communities (68.2%), compared to the SSP with 1.29 communities (64.7%), without significant differences.Species complementarity between SSP and SC fragments was 38.5%, with a species overlap of 61.3%; and a similarityof species of 65%. The fragments of the SSP presented greater equality with 0.70 (95% CI between 0.69-0.73) and less dominance with 0.11 (95% CI between 0.10-0.12) in the bat assembly, compared with the SC fragments.
The high degree of deforestation in the Colombian Caribbean region for the establishment of extensive livestock systems, implies that wildlife must survive in highly fragmented landscapes. New scenarios of extensive livestock with silvopastoral management system (SSP) generates a mosaic with various types of vegetation cover in various succession states, and therefore, a greater heterogeneity of habitats,34,35 and it increasesthe structural complexity of pastures or wooded grasslands,36 which improves functional connectivity and species diversity.37,38 The diversity of registered bats in the bs-T fragments studied, it is comparable with that reported for similar habitats in South America,39–41 and represent the highest record of diversity of bat species associated with bs-T in Córdoba (Colombia), which represents about 35% of the species in Colombia and 58% of the department of Córdoba.42
The greatest richness and abundance of bats found in SSP of extensive livestock, compared to SC fragments, was dominated by the Phyllostomidae family (Table 1) in a similar way to what was recorded for other Neotropic environments.43–46 In the bat guild in SSP, especially of the genus Artibeus, Carollia and Uroderma (Table 1), reflect the greater structural complexity of the vegetation, the high heterogeneity of the mosaic-shaped habitat, the permanent shelters available as tree holes, water passages under roads and roads and increased tree cover in paddocks. Lto presence of pioneering plants of the genera Guazuma, Piper, Cecropia, Vismia, Maclura, Aegiphila and Solanum, increase the supply of resources on the edges of forest fragments, which favor species such as C. perspicillata, typical of intervened environments.47,48
The dominance of C. perspicillata, A. lituratus, A. planirostris, C. castanea and D. phaeotis in the fragments associated with the SSP, is consistent with other studies that have determined their general diet and wide scope of home,46,49 and G. soricina abundance may be related with the ability to forage, and that being a generalist species, can exploit more resources; 45 while heat low abundance of the other species of nectar-eating bats (Table 1) agrees with what was found for other tropical environments with secondary vegetation, and in agricultural production systems, where in general, they are not abundant. 50In bs-T areas the seasonality in flowering and fruiting of plants subjects the species to important variations in food availability,51 with comparative advantages in SSP matrices52 the diversity of foraging areas and habitat quality is higher. These results indicate than SSP management of extensive livestock farming, having better food niche conditions and shelters, positively favors the assembly of bats.
The temporal variation of the bat assembly (Figure 2) is related to the greater supply of fruits in the rainy season of the year,53 environmental condition that generates increases in abundance of fruit bats,54,55 similar to what found for fragmented bs-T ecosystems in Central America.54 Changes in insectivore abundance are related to food availability, which depends on rainfall and primary productivity, positively related in tropical forests, with differences in abundance between the rainy season and the drought.56–58 Naturally abundant generalist species are less susceptible to habitat alteration; whereas, less abundant species are more sensitive to habitat modification.59,60 Thus, eight exclusive species found in fragments of the SSP (Table 1) generally fly within the forest and can live in low, medium or high vegetation.; while the exclusive species found in the SC fragments are generally associated with open areas and sparse vegetation.
The rate of species turnover or true beta diversity between bs-T fragments in SSP and SC from extensive livestock farming (1.24 effective communities) is fundamentally associated with the greater environmental heterogeneity associated with SSP; since, despite the vagility of bats, tree cover determines their distribution and abundance.61 highest rate of species change between climatic periods in SC fragments (Table 2), may cause less stability of the bat assembly. The greater species richness, species abundance and lower rate of temporary change (rain-drought) in the SSP, generates greater stability of the assembly of species and of the ecological processes in which bats participate.
With a similarity of 65% and a complementarity of species of the 38.5% between SSP and SC, the importance of the contribution to regional biodiversity of each fragment of bs-T is evident; therefore, the elimination of any of them would have negative effects on some species of bats. The greater equity in the assembly of bats in SSP is a reflection of the best habitat conditions and greater supply of resources for bats. For its part, the low equity in the assembly of species in the SC with high dominance of very few species (Table 1), is probably a consequence of the decrease in the quality of the habitat, especially in the surrounding matrix of the fragments.62 These results agree with studies carried out for low and high areas of Colombia.63–66 where assemblages of bat species generally present a dominant species.
The type of extensive livestock management in BS-T areas in the Colombian Caribbean generates differential responses in the diversity and structure of the bat assembly. The establishment of SSP increases thehabitat heterogeneity and provide greater supply and availability of resources that favor the conservation of local and regional biodiversity, counteracting the negative effects of livestock SC. The conservation of bs-T fragments associated with SSP, are important elements of the landscape that promote the conservation of bat diversity, and their functional role in the dynamics of tropical ecosystems.
TOValentín Espitia, Liliana Buelvas, Luis Morelos, Ricardo Ortiz Hoyos, Carlos M. González, Mauricio Vela-Vargas, Berta Calonge-Camargo, María Cristina Ríos-Blanco, Elkin León and Helena Olaya for their participation in field and laboratory work.To Paul Betancur, Gustavo Gómez and Salvador Vélez for their interest and logistical support. To the University of Córdoba (Proy.FCB20-10), Pontifical Javeriana University Proy. PUJ-ID 5688) for supporting research funding.q
Authors disclose no commercial associations that might create a conflict of interest in connection with submitted manuscripts.
Authors had no funding for this research.

©2020 Ballesteros, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.