Journal of
eISSN: 2572-8466


Review Article Volume 11 Issue 6
1Department of Chemistry and Industrial Chemistry (DCCI), University of Genoa, Italy
2Department of Bioengineering, University of California, USA
Correspondence: Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA
Received: October 28, 2024 | Published: November 6, 2024
Citation: Tan C, Tawil B. Duchenne muscular dystrophy: current treatments and tissue engineering strategies. J Appl Biotechnol Bioeng. 2024;11(6):177-184. DOI: 10.15406/jabb.2024.11.00375
Duchenne muscular dystrophy (DMD)is an X-linked recessive disorder characterized by progressive muscle degeneration and weakness. Nearly all cases of DMD occur in male children. It remains a challenging condition with no cure, requiring ongoing research and advanced medical care to manage symptoms and improve quality of life. However, the increasing market demand and investment in DMD therapeutics are driven by a more favorable regulatory environment, advances in gene-editing technologies, and significant progress in clinical trial drug developments. Recent clinical trials using cardiosphere-derived cells (CDCs) have shown promise in preventing heart and muscle weakening in non-ambulatory patients, indicating a potential breakthrough in tissue engineering solutions for management of DMD. Furthermore, innovations in tissue engineering, including the use of stem cells and gene therapy are paving the way for novel therapeutic strategies designed for muscle regeneration and functional restoration. This review paper explores healthy muscle physiology, the pathophysiology of DMD and emerging market trends. Furthermore, this paper discusses past and ongoing clinical trials regarding tissue engineering solutions for DMD.
Keywords: muscular dystrophy, tissue engineering, Duchenne muscular dystrophy, muscle degeneration
DMD, duchenne muscular dystrophy; CDC, cardiosphere-derived cell; DAPC, dystrophin-associated protein complex; DCM, dilated cardiomyopathy; pre-mRNA, precursor messenger ribonucleic acid; rRNA, messenger ribosomal ribonucleic acid; ASO, antisense oligonucleotides, SP, muscle side population cells; MPC, muscle precursor cells; TEMC, tissue engineered muscle constructs; AAV, adeno-associated virus; PUL, performance of upper limb; HOPE-2, halt cardiomyopathy progression in duchenne, FDA, Food and Drug Administration
Duchenne muscular dystrophy (DMD) stands as the most prevalent form of muscular dystrophy, primarily affecting male children. This genetic disorder arises from a mutation in the dystrophin gene, prohibiting dystrophin production within the skeletal muscles.1 As individuals with DMD age, they experience rapid progressive muscle weakness, eventually leading to a loss of independent ambulation. Despite ongoing research, there is currently no cure for DMD; treatments such as corticosteroids, gene-editing therapies, and cell-based approaches are available to slow disease progression and alleviate symptoms.2 While life expectancy among those with DMD has improved in recent decades, cardiac and respiratory failures remain the leading causes of mortality2. Many current clinical efforts aim to address the underlying genetic defect by restoring the dystrophin production to improve muscle regeneration and function.
Physiology of skeletal muscle
Skeletal muscle is a structured tissue consisting of bundles of muscle fibers, comprising approximately 40% of human body weight.3 Skeletal muscle contains various nerves and vascular structures alongside metabolic and regulatory machinery to produce energy and maintain cellular homeostasis.4 The striated muscle fibers are multinucleated cells, with the nuclei around the cell’s periphery adjacent to the sarcolemma.5 The sarcolemma functions as a tube-like sheath that surrounds each muscle fiber, creating a boundary between intracellular and extracellular components.5 Each individual muscle fiber contains hundreds and thousands of myofibrils composed of various filaments which play crucial roles in contraction and movement.5 Figure 1 below shows the physiology of a skeletal muscle fiber and its associated components.
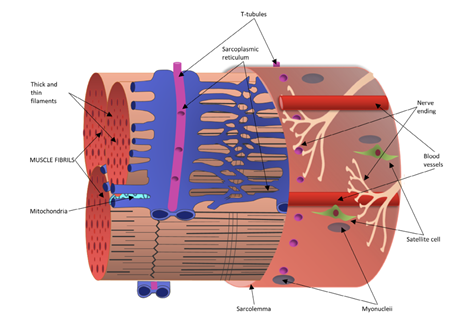
Figure 1 Diagram of skeletal muscle fiber with visible cross-section of a muscle fibril bundle encased by the sarcolemma.55
The image shows a schematic representation of a single, mature skeletal muscle fiber.
Actin (thin filaments) and myosin (thick filaments) are organized in a consistent pattern within sarcomeres, the functional units of muscle, which gives skeletal muscle its characteristic striated appearance. The sarcomeres area longitudinally arranged containing structural components such as the M line, A band, I band, H band, and Z disk.5 In contrast with cardiac muscle, skeletal muscle is unable to generate spontaneous activity due to its lack of ion channels for membrane depolarization. Skeletal muscle activity is instead initiated by a nerve impulse, with most nerve signals being received at central points called motor endplates. Muscle fibers can be categorized into 3 different groups: type I, type IIa, and type IIb. Type I fibers, or slow-twitch fibers, are thinner and meant for sustained endurance activities.6 On the other hand, the thickest type IIb fibers are fast-twitch and best suited for short bursts of intense activity. Type IIa fiber are also considered fast-twitch and have intermediate fatigue rates and myoglobin content. In terms of function, skeletal muscle transforms chemical energy into mechanical energy to produce force and power. It also plays a role in energy metabolism by storing necessary substrates such as amino acids and carbohydrates. While these are characteristics of healthy skeletal muscle tissue, there are a wide variety of genetic muscular dystrophies that degenerate muscle fiber.5 This can cause severe physical weakness in the body, creating difficulties with movement and maintaining body position and posture.5
DMD Pathophysiology
Duchenne muscular dystrophy is an X-linked, severe progressive neuromuscular disorder characterized by degenerating muscle fibers.7 This male-dominant disease is caused by mutations in encoding dystrophin, leading to an absence of dystrophin protein in the muscle.8 Early symptoms of the disease, which are usually present around 2-3 years of age include an unsteady gait, frequent falling, and difficulty running and climbing stairs.9 By the age of 10-12, many patients become wheelchair dependent. Even with preventative care, the life expectancy for DMD patients is around 20-40 years, with most patients dying from cardiac or respiratory failure. The differences in histology between the healthy skeletal muscle tissue and DMD muscle tissue can be seen in Figure 2 below. The diseased tissue contains increases fibrosis, greater variation in myofiber size, and centrally nucleated muscle fibers.8 Degenerating muscle tissue also has a greater presence of inflammatory cells such as macrophages and CD4+ lymphocytes.7
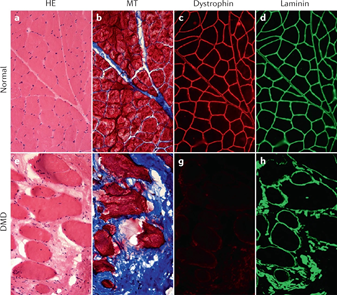
Figure 2 Side-by-side comparison of healthy skeletal muscle tissue and DMD muscle histology.55
The image shows cross-section staining of healthy skeletal muscle (a-d) and skeletal muscle from a patient with DMD (e-h).
The dystrophin deficiency in DMD patients causes the sarcolemma, or the muscle fiber membrane, to weaken.7 In healthy tissue, the sarcolemma needs to manage mechanical stress and force from the contractile activity of the sarcomere. In DMD, the dystrophin-associated protein complex (DAPC) is unstable, resulting in its disassembly.8
Without a functional DAPC, the sarcolemma becomes fragile and is more susceptible to damage during contractions with repeated cycles of muscle fiber damage and repair.8 DMD tissue also produces a greater number of free radicals from reactive oxygen species, making the muscles more prone to oxidative damage and stress.10 Compared to healthy muscle, the resting cytosolic calcium concentration, particularly near the sarcolemma, is much higher.11 The excess calcium leads to mitochondrial dysfunction and metabolic defects11. Degradative pathways such as calcium-dependent calpain protease, phospholipase A2, and mitochondrial-dependent necrosis, are activated, directly contributing to muscle cell death.
Beyond skeletal muscle, DMD also leads to a loss of respiratory muscle strength.12 This leads to respiratory insufficiency and requires treatment through respiratory interventions and support. Patients are typically more susceptible to respiratory viral infections, creating a higher risk for prolonged symptoms such as a cough and eventually chronic respiratory failure.12 Cardiac failure is also a pressing concern, with a high risk of dilated cardiomyopathy, congestive heart failure, and arrhythmias.13 Dilated cardiomyopathy (DCM) is marked by thinning left ventricle walls and progressive left ventricular dilation, indicating a continuous loss of myocytes. The cyclic mechanical stress for the contraction of the heart leads to apoptosis and fibrosis, with the scarring spreading to cause DCM.14 Improvements in respiratory care has improved the outlook for DMD patients but has shifted the primary cause of death towards acute cardiac events and cardiac insufficiency. Early detection and continuous monitoring after diagnosis are important for managing cardiac manifestations of DMD as a more proactive approach can preserve cardiac function and improve patient outcomes.8
Market size
DMD, as the most common childhood myopathy, has an estimated incidence of 1 in 3600 to 1 in 6000 male-born infants.15 As an X-linked recessive disease, females who are carriers of DMD are rarely symptomatic.16 The severity of DMD varies based on mutation, leading to a heterogeneous population of affected individuals.17 In a 2020 systematic review, findings revealed that the median survival age has risen from 18.3 years (95% CI 18.0, 18.9) in a pre-1970 birth cohort to 24.0 years (95% CI 22.8, 25.0) in a 1970-1990 birth cohort, and further to 28.1 years (95% CI 25.1, 30.3) in a post-1990 birth cohort.18 These findings can be seen in Figure 3 below.
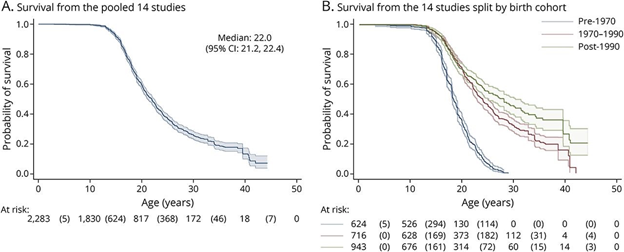
Figure 3 Kaplan-Meier survival curve estimates for DMD patients.18
The image shows the survival curve estimates for DMD patients. A) shows pooled data. B) show data split by birth cohort from the digitized data.
This increase in reported life expectancy can be attributed to improvements in medical disease management as well as use of cardiac and ventilatory support in late stage DMD.19 Between 2016 and 2022, the US healthcare system spent $3.1 billion on eteplirsen, golodirsen, and casimersen–three genetically targeted, exon-skipping drug therapies for DMD.20 As shown in Figure 4, these drug expenditures rose annually from $7 million in 2016 to $879 million in 2022,21 despite fewer than 50,000 people in the US having DMD.22
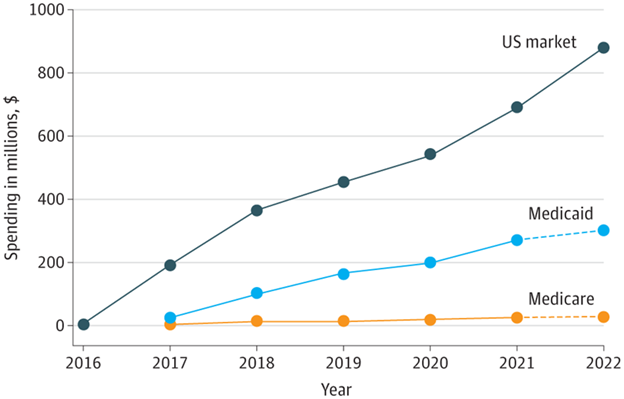
Figure 4 Estimated net US spending on Targeted Therapies for DMD, 2016-2022.20
The image shows the estimated net spending of the US on targeted therapies for DMD, from 2016 to 2022.
DMD has early childhood onset, with affected children usually being diagnosed before 4 years.23 Mortality is low in children between 0 and 10 years, but cardiopulmonary failure is the leading cause of death around the third decade.8
There is no cure for DMD, but the majority of patients are prescribed corticosteroids which have demonstrated some efficacy in delaying disease progression and prolonging ambulation.24 Exon-skipping therapies are another popular treatment option, designed to skip over specific mutated exons in precursor messenger ribonucleic acid (pre-mRNA) splicing to increase dystrophin production.25 As seen in Figure 4 above, federal spending on exon-skipping DMD drugs through Medicaid and Medicare has increased in tandem, from $25 million in 2017 to $327 million in 2022 for a total of $1.2 billion.20
According to a comprehensive global study, estimates suggested that out of 1000 patients between the ages of 20 and 25, approximately 86 fatalities would occur annually. This number rises to 336 for individuals aged over 40 years.18
However, the rarity of DMD and variation in onset severity makes disease prevalence analysis difficult. As patient age rises, data uncertainty grows due to increased censorship and patient mortality.18
Despite the limited availability of data, the market for DMD is continuing to grow due to a rising number of clinical trials and numerous advancements in clinical drug development.26 The DMD market size was valued at $1.03 billion in 2021, with corticosteroid therapy accounting for the largest revenue share.27 There has also been a more favorable regulatory environment driving revenue growth, such as FDA approval of deflazacort corticosteroid tablets in 2017 for patients 5 years and older.27,17 This was the first DMD medication approved for broad patient use and the FDA has currently approved up to four exon-skipping treatments, with many more undergoing clinical and preclinical trials.28
As seen in Figure 5 below, growth is projected throughout the next decade, reaching $11.7 billion in 2033. A restricting factor for market growth is the significant cost of treatment drugs, with eteplirsen estimated to cost over 750,000 annually.26 Current key players at the forefront of DMD therapeutic treatments are Sarepta Therapeutics, Pfizer, Santhera Pharmaceuticals, Eli Lilly and Company, BioMarin Pharmaceuticals, Nippon Shinyaku, and Lexicon Pharmaceuticals.29,27
Market segments and trends
The DMD market can be categorized into therapies related to drugs and small molecules, individual genome, and cells.25 For drug therapies, corticosteroids often show improved muscle function by employing anti-inflammatory and immunomodulatory effects.17 However, the variability of DMD symptoms and its progression results in a lack of consensus on when to start treatment regimens, how long to maintain them, and which therapeutic is most effective.30
Genetic therapies typically utilize exon-skipping approaches, employing artificially synthesized oligonucleotide sequences designed to target specific mutations in the dystrophin gene.25 By inducing the skipping of specific exons during pre-mRNA splicing, these drugs help to restore the genetic reading frame and partial production of the dystrophin protein. Cell therapies generally involve in-vitro cultivation of myogenic cells followed by injection or transplantation into damaged muscle tissues. Table 1 below lists notable therapeutic strategies for DMD listed by treatment, method, advantages, and limitations for each.31–35
|
Treatment |
Method |
Advantages |
Limitations |
|
Corticosteroids |
Anti-inflammatory and immunomodulatory effects |
Stabilize muscle strength and function |
Approximately ⅓ of DMD patients develop vertebral compression fractures, other side effects such as short stature and obesity |
|
Exon-skipping therapies |
Use antisense oligonucleotides (ASO) to target dystrophin mRNA, induce skipping of mutated exons that disrupt dystrophin production |
Helps delay disease progression by increasing dystrophin production |
Exon-skipping therapies are mutation specific (eg. eteplirsen skips exon 51 which affects ~13% of DMD population), not generically helpful for all mutations |
|
Gene replacement with virus vectors |
Use recombinant adeno-associated viruses to replace defective genes |
Successful gene transduction could restore significant function |
Triggers host immune responses, persistent exogenous genetic material in human cells |
|
Nonsense suppression |
Aminoglycosides and agents that suppress nonsense mutations interact with ribosomal RNA units, hindering the recognition of premature stop codons |
Facilitates the translation and synthesis of altered dystrophin protein, restoring muscle functionality |
Long-term use of nonsense mutation suppression agents can result in renal and oto-toxicity |
|
Muscle precursor or stem cell therapy |
Reprogram adult cells with corrected gene and implant healthy muscle stem cells into DMD subjects |
Stabilization of muscle cell deterioration |
No approved standardized procedure for growing and testing cells in human subjects |
Given the absence of a cure for DMD, treatment strategies emphasize enhancing patient growth through dietary management and physical activity.36 Nevertheless, the swift onset of progressive muscle weakness typically culminates in the loss of independent ambulation and often wheelchair dependency by the age of 12.23 Supportive intervention with corticosteroids plays a role in prolonging muscle functionality but associated cardiac and respiratory failures are treated aggressively along with positive pressure ventilation and airway clearance strategies. A 2019 study estimated the medical costs of DMD at $22, 500 annually.17 The annual cost increases with increased age and disease progression due to greater healthcare utilization as DMD patients become non-ambulatory.17 Common commercially available treatment drugs for DMD, both corticosteroid and exon-skipping therapies, are outlined in Table 2 below.37,38
|
Drug name |
Manufacturer |
FDA approval date |
Class of drug |
Method of delivery |
|
Deflazacort |
PTC Therapeutics |
February 2017 |
Corticosteroid |
Oral |
|
Eteplirsen |
Sarepta Therapeutics |
September 2016 |
Exon-skipping of exon 51 |
Intravenous |
|
Golodirsen |
Sarepta Therapeutics |
December 2019 |
Exon-skipping of exon 53 |
Intravenous |
|
Casimersen |
Sarepta Therapeutics |
February 2021 |
Exon-skipping of exon 45 |
Intravenous |
|
Vitolarsen |
Nippon Shinyaku |
July 2020 |
Antisense oligonucleotide for exon 53 |
Intravenous |
Cell-based therapies
Amongst the various strategies designed to restore dystrophin for DMD, stem cell transplantation has the most extensive history, with trials from over 40 years ago.39 These tissue engineering methods to enhance skeletal muscle function have typically focused on cultivating myogenic progenitor cells in vitro, followed by their injection or implantation into damaged muscle (Figure 6).40 Various cell types have been used for these transplantation processes such as mesenchymal stem cells, bone marrow-derived stem cells, satellite myoblasts, and pericytes.
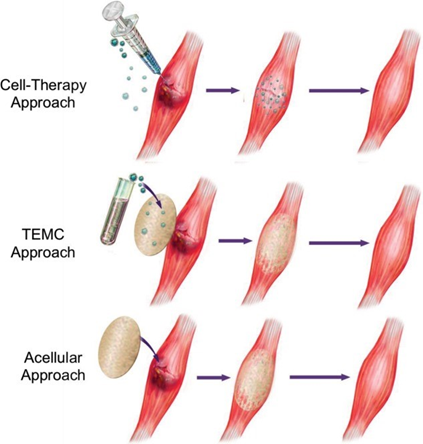
Figure 6 Diagram of different approaches to Muscular Dystrophy Treatment.51
The image shows three approaches for muscular dystrophy treatment: cell-therapy (top), implantable TEMC (middle), and acellular regeneration (bottom).
Initial challenges to early research include a lack of information on specific molecular mechanisms of growth factors responsible for the development of muscle-derived stem cells.41 These cell-based therapies are appealing since transplanted cells have functional copies of the DMD gene, theoretically able to initiate muscle repair upon insertion.39
In practice, these cell transplants are not efficient for treating muscular dystrophy since there are over 700 individual skeletal muscles that compose over 30% of a human’s body mass.39 Tables 3, 4 below outline previous preclinical trials employing cell-therapy approaches in mouse models and clinical trials employing cell-therapy approaches in DMD patients to test cell proliferation capabilities for possible application in DMD application.40
Preclinical cell therapies for DMD (Table 3)
|
Article title |
Cell type |
Transplant method |
Results |
Challenges |
Year |
|
Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts41 |
Myoblasts |
Injection |
Injected myoblasts may integrate with pre-existing mdx-muscle fibers, resulting in elevated levels of dystrophin. |
Necrosis challenges in humans, humans require magnitudes more myoblasts, risks of immune rejection |
1989 |
|
Dermal fibroblasts convert to a myogenic lineage in mdx mouse muscle42 |
Dermal fibroblasts |
Injection |
Abundant levels of dystrophin-positive fibers in mdx-mouse after treatment |
Differences between mice mdx and human DMD are still poorly understood |
1995 |
|
Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice43 |
CD34(+)Sca-1(+) stem cells |
Intra Arterial Injection |
Stem cells administered via intra arterial injection have the ability to migrate to mdx tissues and subsequently contribute to muscle regeneration after injury. |
Specific method of migration to damaged muscles unknown, and can be different in humans as well |
2001 |
|
Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells44 |
muscle side population (SP) cells |
Intravenous Line |
SP cells are capable of traveling through the capillaries to arrive at damaged muscle tissue |
Limited understanding of the analogous system also applies in humans |
2004 |
Table 3 Cell Therapy preclinical DMD trials using muscle-precursor or stem cells in mice models.42– 45
Clinical cell therapies for DMD (Table 4)
|
Article title |
Cell type |
Transplant method |
Results |
Challenges |
Year |
|
Human myoblast transplantation: Preliminary results of 4 cases46 |
Myoblasts |
Injections administered to the tibialis anterior, biceps brachii, and extensor carpi radialis longus muscles |
No symptoms immune rejection despite no immune suppression; strength increases recorded at 0, 41%, 51%, and 143% |
Generally strength increases unremarkable, high outlier increase must be cautiously interpreted due to lack of double-blind strength measuring protocol |
1992 |
|
Myoblast Transfer in the Treatment of Duchenne's Muscular Dystrophy47 |
Myoblasts |
Injections into biceps brachii of one arm of 12 boy patients |
Muscle strength between the two arms did not demonstrate significant differences, and little evidence of donor-derived muscle fiber expression |
Expressions of donor-derived muscle fibers limited to less than 1% in all but one patient, and one with 10.3% expression |
1995 |
|
Dystrophin Expression in Muscles of Duchenne Muscular Dystrophy Patients After High-Density Injections of Normal Myogenic Cells48 |
Muscle precursor cells (MPC) |
Intramuscular allotransplantation |
Percentage of myofibers expression dystrophin from donor ranged between 3.5% and 26%, illustrating improved levels of integration |
Unclear if acute rejection of donor cells was sufficiently repressed by immunosuppression methods, and expression restricted to injection sites. |
2006 |
|
Autologous Transplantation of Muscle-Derived CD133+ Stem Cells in Duchenne Muscle Patients49 |
Muscle-derived CD133+ cells |
Left abductor digiti minimi muscle injections |
No significant side effects of therapy; treated patients recorded elevated ratio of capillaries per muscle fiber, and transition to fast myosin-containing myofibers |
Unclear biological justification for switch from slow to fast muscle fiber type |
2007 |
Table 4 Cell therapy clinical DMD trials using muscle-precursor or stem cells in human models.46– 49
Limitations and challenges of cell-based DMD therapies
These attempts, although extensive, were deemed unsuccessful for in vivo applications due to several factors, including limited viability of donor cells following transplantation as well as induced immune response with ineffective immunosuppressive therapies.50 In particular, bone marrow-derived cells, pericytes, and mesenchymal stem cells have limited capacity to enter muscle tissue and promote myogenesis. Induced pluripotent cells differentiated into myogenic lineage do not have these proliferation challenges due to greater differentiation potentials, but there is an additional risk of uncontrolled growth and the development of teratomas.39
To facilitate cell proliferation, biologic scaffolds have been utilized in conjunction with myogenic cells and their precursors, aiming to stimulate a regenerative muscle response.37 This is accompanied by the development of implantable tissue engineered muscle constructs (TEMCs) which require ex-vivo culture for effective cell-scaffold integration.37 However, the process of in vitro culture or ex vivo culture followed by transplantation of myoblasts for muscle dystrophy has many technical challenges.51 For proper biocompatibility and function, engineered TEMCs must integrate with host tissue through both neuromuscular innervation and functional vascularization. Cell-therapy strategies, such as these, are difficult to pursue since they typically maintain higher costs and regulatory standards for the manufacturer.
Another regenerative approach entails the use of decellularized allogeneic or xenogeneic mammalian tissue. These acellular tissues serve as the foundation for creating inductive bioscaffolds made from extracellular matrix, thereby encouraging the recruitment of endogenous progenitor cells to the site of injury for subsequent healing. The cell therapy, implantable TEMC, and acellular approaches discussed above can be seen in Figure 7 below.51
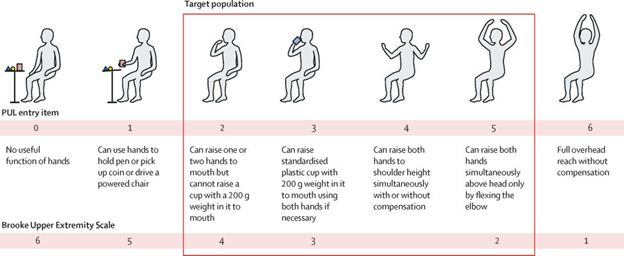
Figure 7 Performance of Upper Limb (PUL) entry items.54
The image shows performance of upper limb entry items for HOPE-2-trials.
Tables 3, 4 above highlight previously completed pre-clinical and clinical trials to test the efficacy and feasibility of cell therapies to treat DMD. Yet, recent therapeutic endeavors have a stronger shift towards gene transfer therapy utilizing adeno-associated virus (AAV) vectors and exon skipping agents.52 AAV treatments have limited efficacy since previous infection with an AAV wild-type is fairly common, leading to the presence of AAV transduction neutralizing antibodies in around 60% of individuals.53 The clinical advantage of exon skipping agents, unlike corticosteroid therapies, includes their ability to slow DMD progression and relieve secondary symptoms. However, existing exon-skipping therapies approved for exons 45, 51, and 53 are only applicable to approximately 30% of all patients with DMD.54 Notably, clinical evidence validating their efficacy in preserving upper limb function is lacking.38
Ongoing clinical trials
A recent development in cellular therapeutics is in the progress of Halt Cardiomyopathy Progression in Duchenne (HOPE-2), the first systemic cell therapy trial that works to address some of these challenges. In phase 2 of the trial, subsequent intravenous infusions of allogeneic cardiosphere-derived cells, or CDCs, were injected into patients.54 The objective was to evaluate both the safety and efficacy of the treatment utilizing the performance of upper limb version 1.2 (PUL 1.2) score following a 12 month period54. In the treatment group, the mean 12 month change for PUL1.2 score favored CAP-1002, or the CDCs, over the placebo54. The PUL entry items used during the study can be seen in the figure below.
CDCs are progenitor cells that have been previously shown to demonstrate immunomodulatory and regenerative behavior in cases of muscular dystrophy and heart failure.55 This occurs through the secretion of exosomes that target macrophages to adopt a healing, non-inflammatory response. Phase 1 of this trial, HOPE-1, determined an acceptable safety and efficacy in preserving upper limb and cardiac function in 13 DMD patients after a singular infusion into the coronary arteries.
The HOPE-2 trial focused on non-ambulatory patients due to their limited treatment options and severe muscular deterioration. The following parameters were used to demonstrate favorable outcomes: MRI imaging of cardiac muscle, spirometry to measure respiratory function, the PUL motor function scale, and the presence of biomarkers.55
Encouragingly, this therapeutic candidate has demonstrated safety and regenerative biological effects in patients with late-stage DMD, motivating a larger, longer-term Phase III clinical trial, HOPE-3. Next steps involve gaining drug approval for CAP-1002 to treat cardiac and skeletal muscular degeneration. This needs to be proven in DMD patients beyond a 12 month period. Additional trials will also need to be performed on an ambulatory DMD patient pool to assess associated efficacy and risks. This trial brings clinical significance since it is the first therapeutic trial to demonstrate increased stability with regards to upper limb mobility and alongside strengthening of cardiac structure.55
While DMD is a rare disease, its debilitating symptoms and the lack of a substantive treatment are continuing to drive clinical research towards a cure.54 With significant progress in the recent HOPE-2 trials, the desirability for a safe and effective DMD treatment continues to grow. Existing treatment methods, such as corticosteroids and exon-skipping drugs have long-term side effects and limited efficacy for the wider patient population due to the individual-specific progression and development of DMD.27 Skeletal muscle and cardiac muscle strengthening must be achieved to allow for treatment of progressive loss of mobility and cardio-respiratory failure.2 Researchers must soon transition to long-term trials in order to prove therapeutic efficacy for the novel HOPE trials and gain FDA approval for widespread treatment use. Despite the current scientific limitations, there is progress being made towards more robust treatments in multiple designations, from cell-based therapies to AAV gene-editing.33,50
Charmaine Tan expresses appreciation to Professor Bill Tawil for overseeing the framework of this review, and for the insightful lectures and advice concerning biomaterials and tissue engineering, which contributed to the development of this paper.
None.
Authors declare that there is no conflict of interest.

©2024 Tan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.