Journal of
eISSN: 2572-8466


Review Article Volume 10 Issue 4
Department of Bioengineering, University of California Los Angeles, USA
Correspondence: Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA
Received: July 05, 2023 | Published: August 9, 2023
Citation: Lina K, Bill T. Diabetic foot ulcers: physiology, disease, market analysis, treatments. J Appl Biotechnol Bioeng. 2023;10(3):101-111. DOI: 10.15406/jabb.2023.10.00335
Diabetic foot ulcers (DFU) are a common, yet preventable complication of diabetes that can lead to serious health risks if left untreated. DFUs pose a significant challenge on not only the affected individual but also the community due to their complex pathophysiology and limited, often insufficient treatment options. This paper will provide a holistic review on diabetic foot ulcers, elaborating on their physiology, the current and projected market sizes, and discussing available treatments and products. Tissue engineering is becoming a quite impactful option for treating diabetic wounds, and is the central focus of this review. Additionally, this paper presents pipeline products, as well as a novel product that utilizes the core concepts of regenerative medicine and innovative technology and thus has the potential to overcome many of the limitations associated with existing therapies. Overall, this review serves as a valuable resource for healthcare professionals and researchers interested in diabetic foot ulcers, offering insights into the current state of knowledge and presenting a promising approach that could revolutionize the field.
Diabetes Mellitus (DM) has become one of the top 10 causes of death worldwide, with a 70% increase from 2000, and it still on the rise (Figure 1).1 The most common type is type 2 DM (T2DM) , which accounts for 90% of all cases, and is characterized by insulin resistance, and therefore hyperglycemia.2 While T2DM is most common in people above the age of 45, the disease is becoming more common in younger populations as well due to rising trends in obesity, unhealthy diets, and lack of sufficient exercise.2 Common complications due to diabetes are ischemia and diabetic neuropathy (DN), and around 60% of people with DN will develop diabetic foot ulcers (DFU), which will often go unnoticed for prolonged periods due to the extensive nerve damage.3 Current treatments include offloading, debridement, and glycemic control, yet these all have limitations due to risk of infection, cost, inconvenience, and likelihood of recurrence of foot ulcers.1 Therefore, advanced therapies using regenerative medicine techniques are becoming a more popular potential solution to allow for better wound healing and more effective prevention of DFU recurrence.1

Figure 1 Number of adults (ages 20-79 years) with diabetes worldwide .15
Physiology of normal wound healing
Wound healing is a multifaceted biological reaction to tissue injury, comprising three key phases: the initial inflammatory stage, the proliferative phase, and the subsequent maturation and remodeling stage (Figure 2).4 During the inflammatory phase, which typically lasts for several days, many critical processes occur.4 Hemostasis occurs for 5-10 minutes, where vasoconstriction and formation of the platelet plug occurs to stop bleeding, then the coagulation cascade occurs which leads to fibrin clot formation.4 Neutrophils and macrophages are critical components for killing and removing debris and bacteria and macrophages secrete growth factors necessary for tissue repair.5 This stage helps to prevent further damage, close the wound, remove debris and bacteria, and promote migration of cells.4 In the proliferative phase, normally lasting several weeks, granulation tissue forms, reepithelialization occurs, and new blood vessels are formed (neovascularization).4 Fibroblasts are responsible for laying down the extracellular components, and will also stimulate collagen production.5 At the end of this stage, the wound bed would be covered with new epithelium and a scar will be apparent.5 The remodeling stage is characterized by ongoing synthesis of collagen to strengthen the tissue, and the wound contracts leading to increased strength.5 However, this area will not be as strong as the uninjured skin and is thus at risk of opening again.5
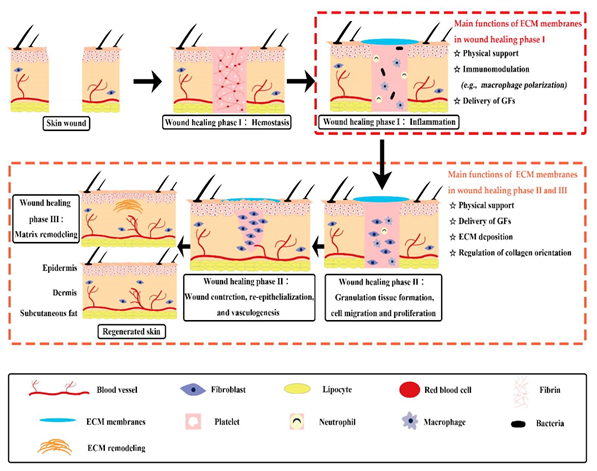
Figure 2 Stages of wound healing.6
The skin’s functions of not only wound healing, but thermal regulation, tactile sensations, and pain sensations are performed by 3 tissue layers. The thin layer on the outside is the epidermis, while the thicker layer in the middle is the dermis. The innermost layer is the subcutaneous layer, also known as the hypodermis by Alexander Reyzelman. The epidermis is made up of keratinocytes that serve as a barrier to prevent loss of water and entry of microbes, and keratinocytes are responsible for reepithelialization. The dermis is connective tissue made up of mainly of collagen and elastic fibers that are produced by fibroblasts, and the dermis makes up a significant portion of the ECM. These two layers contain blood vessels, nerves, sweat glands, hair follicles, and cells that allow for immune function, growth, and repair of tissue, while overall giving the skin its strength and allowing for diffusion of nutrients and water. The hypodermis consists of a thick stratum of adipose tissue (Figure 3).
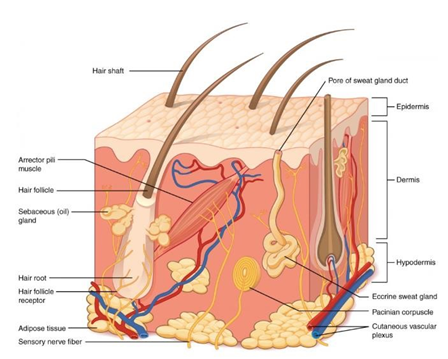
Figure 3 Layers of the skin.7
Physiology of wound healing of the diabetic foot
The main complication of DM is impaired wound healing abilities due to a complicated pathophysiology that involves vascular, neuropathic, biochemical and immune pathways (Figure 4).8,9 Hyperglycemia is often associated with stiffer blood vessels, so circulation is slower, causing tissues, particularly in the extremities, to get less oxygen and can lead to higher risk of infection.9 Peripheral neuropathy can cause numbness to affected areas, so chronic wounds could go unnoticed.8,9 Also, DM can lead to deformities in the foot that increase plantar pressure and dryness, which furthers the risk of getting wounds.8,9

Figure 4 Comparison of normal wound healing (left), with diabetic wound healing process (right).8
For people with DM, the inflammatory conditions become chronic, as angiogenesis is disrupted, there are less endothelial progenitor cells, and ECM regulation is irregular.8 While neutrophils and macrophages still enter the wounded area, some cytokines such as IL-1ꞵ and TNF⍺ are elevated for extended amounts of time in people with DM.8,10 Other growth factors (IGF-1, TGFꞵ) involved in processes of cell-granulation, wound re-epithelialization, angiogenesis, and ECM formation are reduced in diabetics.8 Hyperglycemia leads to less amounts of VEGF, and macrophages have deformed phenotypes, so proper tissue repair does not occur.8,11 The remodeling phase is also affected by diabetes, as factors that help form the mature vasculature phenotype are reduced, thus further decreasing wound healing.8,12 An imbalance of MMPs and TIMPs in people with DM alters the ECM regulation, so the scaffold structures are disrupted.8 These reasons are why many diabetic patients develop DFU, and many become so infected that osteomyelitis occurs. Also, once an ulcer forms, the rate of recurrence becomes higher, so it can be difficult to find treatments that significantly decrease the incidence of DFU.13
There is a spectrum of severity of DFU, and there are 6 classifications (Figure 5).14 Grade 0 is associated with foot symptoms, such as pain, and there is no clear wound yet.14 Grade 1 corresponds to presence of superficial ulcers and progression to grade 2 is characterized by deep ulcers. Grade 3 is when the wound has now involved the bone, and tendons and muscles will be exposed, and risk of infection is high. At grade 4, presence of dead tissue or gangrene is evident, particularly in the forefoot. In grade 5, the most severe grade, the entire foot is gangrenous.14
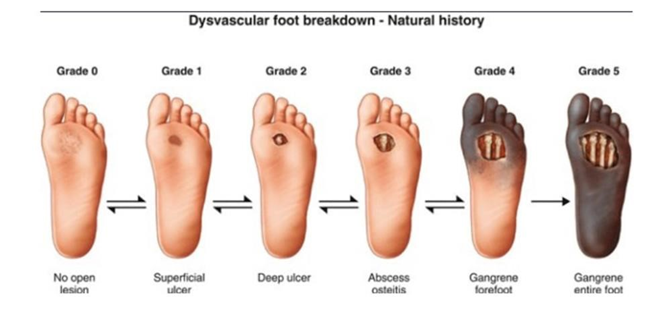
Figure 5 Grades of diabetic foot ulcers.14
Market size, segments, and trends
Around 537 million adults are living with diabetes, and this number is projected to rise to 783 million by 2043.15 The global DFU market size was around 7.03 billion USD in 2019, and is forecasted to reach 11.07 billion in the next 5 years.16 Some main driving factors that are contributing to this growth in the market is not only increases in diabetes but increases in complications of the disease due to inactive lifestyles, age, and longer duration of DM.17,18 The growing DFU treatment market is mainly due to increasing prevalence of foot ulcers as well as larger scale implementation of new wound care devices.16 The geriatric population is increasing, and demand for new DFU therapies continues to escalate.2,16 The global prevalence of DFU is 6.3%, with North America having the highest incidence, of 13% of worldwide DFU cases (Figure 6).17
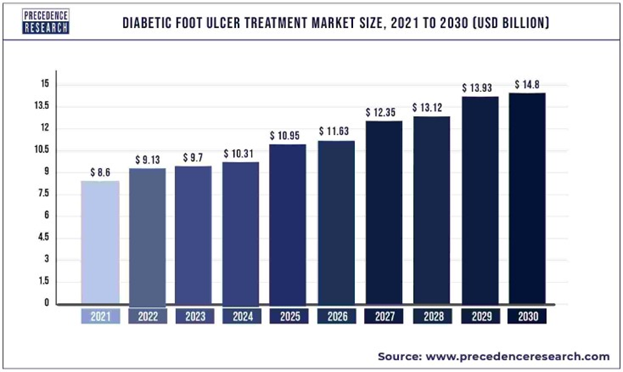
Figure 6 Global diabetic foot ulcer treatment market size, 2021 to 2030.18
The United States has spent over 966 billion dollars on healthcare specifically related to diabetes, which corresponds to an increase of 316% over the past 15 years.15 North America dominates the market share due to rising prevalence of DFU as well as large healthcare expenditure, advanced treatment options, and expensive products. Asia Pacific recently became the second largest share, as it increased its rank since 2019, when it used to be third highest.16,18 This sudden increase is due to the geriatric population in this region rising at an unprecedented rate, as well as rapid urbanization leading to more sedentary lifestyles.16,18
While Latin America, the Middle East, and Africa will experience significant rises in diabetes, they will not experience nearly as high compound annual growth rates (CAGR) due to increasing unmet needs for affordable and effective treatment options for DFU and developing healthcare systems (Figure 7).16
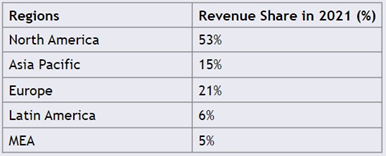
Figure 7 DFU treatment market share by region, 2021.18
The market is also segmented based on ulcer type, where the three types are ischemic ulcer, neuropathic ulcer, and neuroischemic ulcer.16 Ischemic ulcers are primarily caused by insufficient blood flow to the foot, while neuropathic ulcers are due to nerve damage, so sensation is lost in the foot and can lead to damage.19 Neurosichemic falls in between the two.19 In 2021, the neuroischemic ulcers segment held the largest market share in the industry, accounting for over 50% (Figure 8).20 The market for this ulcer type is also expected to grow, likely due to the high risks related to neuroischemic ulcers, including infection, amputation, and mortality.46 In addition, neuro-ischemia affects around 50% of diabetics, so neuroischemic ulcers are the most prevalent type of DFU, and have higher associated costs of treatment.16,20 As an example, the National Center for Biotechnology Information wrote that the cost of ambulatory care treatment for a neuropathic ulcer is below 60 USD, while expenses for a bypass surgery for neuroischemic ulcer are almost 2000 USD.16,21 The segment of the market for ischemic DFU is expected to decrease, as there is a decrease in cases of purely ischemic DFU.20
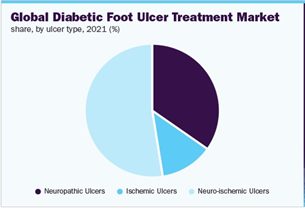
Figure 8 Global DFU treatment market by ulcer type.46
Considering product type, the segment of wound care dressings is expected to be the market leader (Figure 9).16 A few primary reasons to explain this trend is increasing focus on R&D market players, increased launches and escalating awareness of advanced products, and soaring rates of healthcare expenditure.16 Another segment that is expended to grow considerably is that of wound care devices, as new therapeutic devices such as negative pressure wound therapy and extracorporeal shock wave therapy are becoming more popular.16 Wound care devices allow for reduced healing time at lower costs, so this is likely a contributing factor to their growing appeal.16 The active therapies segment also has a significant share in the market, and is also expected to increase.16 This segment includes skin substitutes and skin grafts, growth factors, sealants, and hemostatic agents.16 Novel applications of these treatment options, as well as more existing alternatives are expected to cause this segment to grow.16

Figure 9 Global DFU treatment market by product Type.16
Looking instead at end-user outlook, the DFU treatment market segments are hospitals, clinics, ambulatory surgical centers, and home care settings.16 Clinical and home-care settings held a revenue share of over 50% in 2021.20 The hospitals segment has the second largest revenue share in 2021.20 A rise is expected in this segment due to adoption of more treatment methods in hospitals, the necessity of trained staff to apply such treatments, and rising patient preference to hospitals.16 However, the segment anticipated to have the largest growth rate is the homecare setting due to the rise in geriatric population and a shift in preference to self-care at home.16 The clinical setting segment is also expected to grow at a significant rate due to a rising number of specialty clinics that have trained staff, high-tech facilities, and personalized wound care treatment options.16 Additionally, due to Covid-19, there was a considerable shift towards teleconferencing, allowing for many clinics to treat DFU patients virtually via digital photography and online communication.20 This is another contributor to the increased growth rates of both clinic and at-home segments.20 Some current companies dominating the DFU treatment market are Smith and Nephew, Integra Lifesciences, ConvaTec Group, 3M, and Tissue Regenix, among others.16-32
Current treatments and products
Current Standard of Care (SOC) protocol for chronic wounds has 5 main steps (Figure 10).33,22 First, carefully clean the area and remove the necrotic tissue in the area through debridement.33. Then, to control exudate and keep moisture balance, apply the appropriate wound dressing, as there are different options for different types and grades of DFU. Third, measures must be taken to prevent infections at the wound site, or prevent any if they arise.33 Finally, offloading must be applied to keep pressure off the wound.33 During the wound healing process, blood glucose should be managed and measures should be taken to ensure sufficient blood perfusion so the wound can heal properly.22
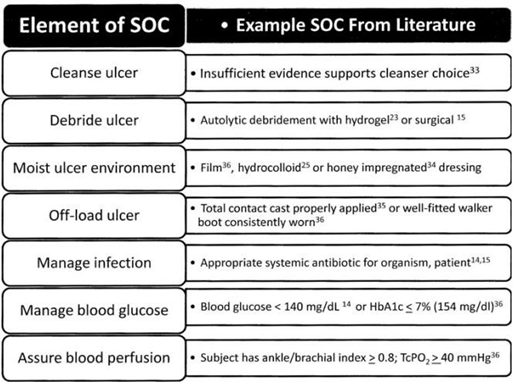
Figure 10 A generic SOC based on FDA guidelines, American diabetes association, and International working group on the diabetic foot.48
Some of the main options for wound dressings include hydrogels, hydrocolloids, acrylics, calcium alginates, and foams.19 If the wound has a lot of fluid secretion, then absorbent dressing are necessary; otherwise, moisture balance dressings are needed to provide moisture to dry wounds.19 If, after 4 weeks of using the SOC approach, wound size has not reduced by around 50%, then alternative therapies or more advanced treatment options might be necessary, as the probability of the wound healing by 12 weeks becomes less than 9%.33 In addition to SOC, some non-tissue engineering FDA-approved treatments for DFU are summarized in Table 1. These therapies all work to contribute to better wound healing, through stimulating different components necessary for effective wound healing, with the hopes of decreasing the size of the wound and improving the healing process while avoiding infection (Figure 11).19
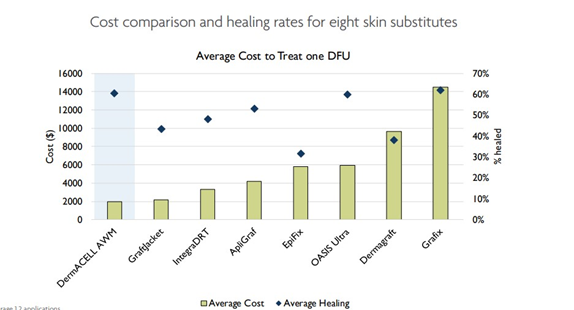
Figure 11 Cost Comparison and healing rates for 8 skin substitutes for DFU, as of 2020.31
|
Type of Therapy |
Pharmaceutical Form |
Route of Administration |
Advantages |
Limitations |
|
Becaplermin |
Gel |
Topical |
Stimulates different growth factors useful in the treatment of DFUs |
Short half-life time |
|
Cell therapy |
Injection or gel |
Locally |
Stimulates different cellular mechanisms for the regeneration of chronic wounds |
Short half-life time |
|
Collagenase clostridial |
Ointment |
Topical |
Easy application, minimal blood loss, and proliferation of endothelial tissue |
Burning, exudation, and inflammation |
|
Dermapace system |
Device |
Local shock waves |
Stimulates the wound mechanically, for the removal of damaged tissue |
Secondary side effects (pain, bruises, etc.) |
|
Deferoxamine |
Injectable |
Locally |
Reduction of ulcers area in less time |
Adverse reactions and its lifetime is short |
|
Granulox |
Spray |
Topical |
Accelerating the healing of chronic wounds |
Short half-life time |
|
Omnigraft |
Device |
Topical |
Potential for improvement in the DFU |
New infections, swelling, and new ulcers, or existing ulcers that would worsen |
|
Piperacillin/tazobactam (Zosyn, Pfizer) |
Injectable |
Locally |
Wide spectrum advantage in infections and low nephrotoxicity |
Adverse reactions may include diarrhea |
|
Provant |
Device |
Locally |
It is useful in pressure ulcers |
There is little evidence of its efficacy |
Table 1 DFU therapies that are Food and Drug Administration (FDA)-approved
While there are topical agents and many different wound dressings, some novel therapies include hyperbaric oxygen therapy (HBOT) and negative pressure wound therapy (NPWT).20 HBOT consists of a person entering a pressurized room with almost pure oxygen, which raises the amount of oxygen flowing into the blood and thus arriving at the wound site. HBOT is FDA-approved and has clinical evidence that suggests its efficacy in promoting wound healing. NPWT is a device made up of a dressing, tubing, and a suction pump, and it utilizes suction to increase blood flow, promote angiogenesis, clear bacteria, and reduce oedema.34–50 Newer treatment options are adopting tissue engineering approaches, using scaffolds and stem cells (Figure 12).20
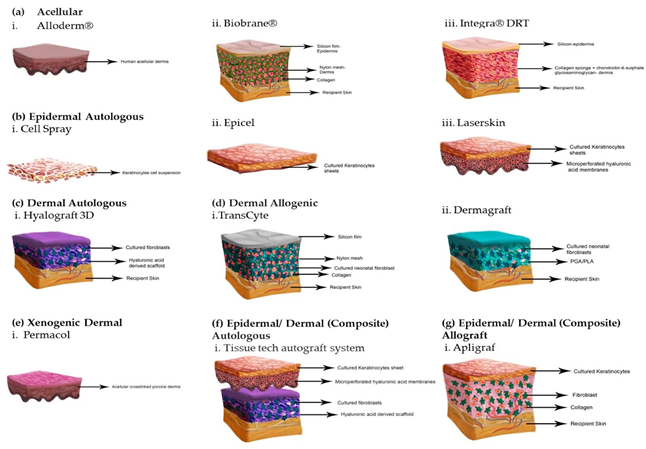
Figure 12 Tissue engineered skin substitutes.42
Some innovative biological products used for treatment of DFU include grafting, culturing fibroblasts and/or keratinocytes, bovine fluid collagen, cell dermal matrix, and human amniotic membrane.19 The actions of each of these are summarized in Table 2, and they are similar in that they are central to the more advanced, albeit more expensive options on the market for treating non-healing wounds of the foot.19
|
Biological product |
Action |
|
Grafting (bioengineering) |
Promotes wound healing through the addition of extracellular matrices that induce growth factors and cytokines |
|
Culture of fibroblasts |
Creates a three-dimensional dermis that is replaced as a graft; |
|
Culture of |
it is used to treat non-ischemic ulcers |
|
fibroblasts/keratinocytes |
Creates a three-dimensional dermis that replaced as a graft; it is used to treat non-ischemic and ischemic ulcers It is a well-refined fluid fibrillar bovine collagen; unlike |
|
Bovine fluid collagen |
normal collagen in biological scaffolds (cross-linked collagen), it contains fibrillar collagen, that is, non-cross-linked collagen |
|
Cell dermal matrix |
It was used for several years for wound healing, tissue repair, and reconstruction |
|
Human amniotic membrane |
It is used as wound coverage |
Table 2 Biological product and its action
Different strategies for wound treatment include skin grafting with autografts, skin allografts, xenografts, and amnion.42 Skin grafting with an autograft is used for deep chronic wounds, as there are not enough keratinocytes to repair the epithelium.42 A split-thickness graft, which is a thin layer of skin including the epidermis and a part of the dermis, is shaved from the donor and placed onto the wound site (Figure 13).42 Autografts have no risk of rejection, as they use the patient’s own skin.42 Allografts often come from cadaveric sources, and they are a good option as they revascularize, provide a barrier, promote angiogenesis, growth factors, and cytokines to further promote wound healing.42 Allografts act as temporary covers, so a further skin autograft is often used to allow for wound closure.42 Xenografts are grafts obtained from a different species (offen porcine), and they incorporate exogenous collagen into the wound site, and get absorbed as the wound heals.42 Amnion is another great option, and is usually collected from placentae.42 Unique benefits of amnion are that it decreases loss of protein, electrolytes and fluids, lessens pain, lowers infection risk, speeds up healing, and can even diminish scar formation.42 Cell-based treatments are also becoming a growing option, especially using cells like human dermal fibroblasts, keratinocytes, and bone marrow-derived mesenchymal stem cells (Figure 14).42
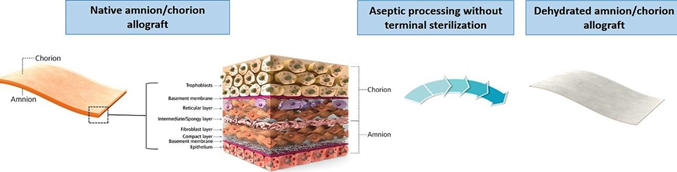
Figure 13 Amnioband structure and appearance.43

Figure 14 Comparison of human skin and Theraskin.52
Skin substitutes are an effective alternative to using skin grafts, as they can serve as temporary covers to protect the wound bed and accelerate wound healing through releasing growth factors and cytokines.42 Ideally, a skin substitute should be sterile, nontoxic, have a low inflammatory response, allow for water vapor transmission, and act as a protective barrier.51 They must also adhere to the surface of the wound quickly, undergo controlled degradation, have sufficient structural properties, be easy to handle, conform to any wound surface, facilitate wound healing and vascularization, be inexpensive, have a long shelf-life, and have convenient storage options (Figure 15).51 While these are difficult requirements, many novel products are being made and studied that are paving the way for skin substitutes to potentially become a commonly available option.51 While the goal of skin substitutes is to model the structure and function of the normal skin, no substitute will completely replicate the skin, but there are many advanced options (Figure 16).
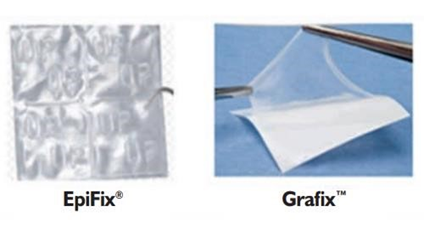
Figure 15 Epifix and Grafix.53
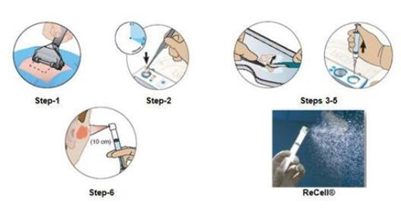
Figure 16 Preparation of spray for regenercell.58
Skin substitutes can be categorized into various types based on their characteristics.42 These classifications include permanent, semi-permanent, or temporary substitutes, as well as epidermal, dermal, or composite substitutes.42 They can also be categorized as cellular or acellular, and further divided into biological substitutes such as autologous, allogenic, or xenogenic, and synthetic substitutes such as biodegradable or non-biodegradable materials.42 The main purpose of synthetic acellular skin substitutes, such as Integra, is to act as barriers to prevent loss of fluids and prevent microbial contamination.42 Acellular options often consist of a collagen layer acting as the dermis, while a silicone layer acts as the epidermis.42 Graftjacket and Dermacell are allograft acellular options (Figure 17).28,30
Figure 17 Palingen Flow.69
Natural skin substitutes mainly involve allogenic or autologous cells, and sometimes also include a dermal matrix.42 Some examples of tissue engineered skin with allogeneic cells are Apligraf, Dermagraft, and Theraskin.25,42 TissueTech Autograft System is an example of a composite autologous substitute, and Apligraf is a composite allograft substitute, as both of these options include a dermal and epidermal layer.21,41,42 Oasis is a xenograft as it is derived from porcine material.32 Epifix, Amnioband, and Grafix are all derived from the placenta.33,38,43 A detailed summary of each of these options is included in Table 3.
|
Stem cell type |
Advantages |
Disadvantages |
Clinical studies |
Preclinical studies |
|||
|
Adult stem cells |
BM-MSC |
• Donor-specific therapy |
• Cell lineage committed (limited differentiation potential) |
19 |
-52.80% |
27 |
-50.00% |
|
• Lower malignancy risk |
• Biopsy high surgical risk |
||||||
|
• Cell-lineage committed (targeting differentiation) |
• Nondisposable tissue |
||||||
|
• No ethical conflict |
• Low stem cell concentration |
||||||
|
• Cell concentration and performance influenced by comorbidities |
|||||||
|
PB-MSC |
• Donor-specific therapy |
• Cell lineage committed (limited differentiation potential) |
11 |
-30.50% |
2 |
-3.70% |
|
|
• Lower malignancy risk |
• Cell concentration and performance influenced by comorbidities |
||||||
|
• Cell-lineage committed (targeting differentiation) |
• G-CSF administration needed |
||||||
|
• No ethical conflict |
|||||||
|
• Relatively disposable tissue |
|||||||
|
• Vein puncture has low surgical risk |
|||||||
|
• Simple cell harvesting protocol |
|||||||
|
hUC-MSC |
• Future donor-specific therapy |
• Cell lineage committed (limited differentiation potential) |
4 |
-11.10% |
12 |
-22.20% |
|
|
• Lower malignancy risk |
• Immunoincompatibility |
||||||
|
• Cell-lineage committed (targeting differentiation) |
• Ethical conflict |
||||||
|
• Disposable tissue |
• Low stem cell concentration |
||||||
|
• UC tissue harvesting has low surgical risk |
• Need for UCB banking |
||||||
|
• Donor UCB banking storage |
|||||||
|
ADSC |
• Donor-specific therapy |
• Cell lineage committed (limited differentiation potential) |
3 |
-8.30% |
11 |
-20.40% |
|
|
• Lower malignancy risk |
• Cell concentration and performance influenced by comorbidities |
||||||
|
• Cell-lineage committed (targeting differentiation) |
|||||||
|
• No ethical conflict |
|||||||
|
• Disposable tissue |
|||||||
|
|
|
• Liposuction has low surgical risk |
|
|
|||
Table 3 Stem cell types advantages, disadvantages and use in clinical and preclinical studies
The commercially available skin substitutes have some limitations, including lower vascularization, insufficient mechanical strength, scarring, rejection, and failure of integration.51 In addition, the process to prepare these substitutes is often quite expensive and time-intensive.51 More cells would also need to be incorporated into many of the available grafts in order to better mimic the skin and be able to form differentiated structures.51 While there is still a long way to go before such products can better mimic the healthy skin and be affordable, research is advancing constantly in this field and options are bound to improve (Figure 18).52,53
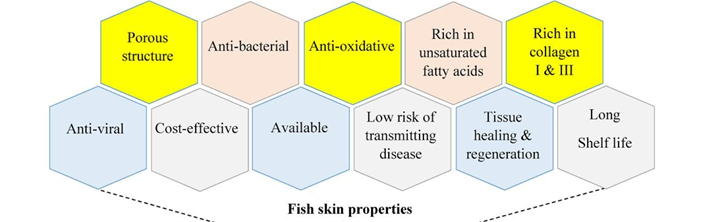
Figure 18 Benefits of acellular fish scaffolds currently in use.72
Pipeline products- in development and clinical trials
In addition to the current products on the market, many more are being tested in both pre-clinical and clinical studies. One of the main treatments that are gaining traction is the use of stem cell based therapies, as stem cells make and secrete cytokines that promote cell migration to the area, immunomodulation, remodeling of the ECM, angiogenesis, and they can differentiate into many different types of cells necessary for proper wound healing.54 Bone-marrow derived mesenchymal stem cells (BM-MSCs), human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) and adipose-derived stem cells (ADSCs) are the specific stem cells used in most current preclinical studies for potential treatment of DFU.54 While many of these studies involve injections, they could potentially be implemented into scaffolds for treating DFU.54
Granulocyte-colony stimulating factor (GCSF) is a cytokine that is crucial to wound healing as it promotes mobilization of endothelial progenitor cells, so many trials are testing to see if there is an optimal dosage of GSCF that can promote wound healing.54 A detailed description of stem cells of interest for DFU treatment is included in Table 4.
|
Pipeline product |
Responsible Party |
Composition |
Potential advantages |
Potential |
Phase of clinical trial |
|
|
|
|
|
disadvantages |
|
|
ReGenerCell |
Avita Medical |
Patient's own skin cells |
-Autologous |
-May be more inconvenient than other options on the market |
Phase 4 |
|
Autologous Cell |
-Prepared and applied in |
||||
|
Harvesting |
at least 30 min[57] |
||||
|
Device56 |
-Spray |
||||
|
ExpressGraft-C9T1 |
Mallinckrodt |
Fully-stratified |
-Engineered with cathelicidin[60], antimicrobial |
- |
Phase 1 |
|
Skin Tissue59 |
epithelial layer of |
||||
|
human |
|||||
|
keratinocytes Dermal layer of fibroblasts |
|||||
|
allogeneic ADSCs |
Medical University of Warsaw |
Adipose- derived |
-Differentiation of proteins involved in wound healing[62] |
-Requires freezing, thawing, then culturing |
Phase 1 |
|
in fibrin gel61 |
stem cell Fibrin gel |
-Can assess cell viability before application |
|||
|
Cell2Cure63 |
Cell2Cure |
Adipose tissue derived mesenchymal stromal/stem cells |
-Can be mass produced, one donor sample can make 50-200 doses[64] -ACSs promote wound healing |
-Cryopreserved -Requires 5-10 minutes of thawing |
Phase 1 |
|
ApS |
|||||
|
TTAX0165 |
Tissue Tech |
Human amniotic membrane and umbilical Cord |
-Can be used for cases with severe osteomyelitis, and exposed tendon, joint, bone[67] |
-Cryopreserved |
Phase 3 |
|
(AM/UC) |
|||||
|
matrix[66] |
|||||
|
PalinGen Flow |
Amnio |
Human amniotic tissue allograft |
-Liquid |
-Not meant for |
Phase 2 |
|
Amniotic Tissue |
Technology |
-Used with SOC, could be alternative to grafts |
more severe forms of DFU |
||
|
Allograft68 |
|||||
|
Kerecis Dura70 |
Kerecis |
Acellular fish skin |
-Piscine, no risk of disease transmission[71] -Promotes more migration into scaffold than amnion/ chorion grafts |
-Could be harder to control degradation rate due to larger pore size |
- |
|
-Faster healing than with porcine graft |
|||||
|
-No cultural constraints on usage[70] |
|||||
|
-Thick sheets, often only |
|||||
|
|
|
|
one necessary -Cost effective -Processed to remove proteins that cause allergies |
||
Table 4 Pipeline products advantages, disadvantages
In addition to testing different stem cells that can be used in potential products, there are many studies determining the safety and efficacy of various pipeline products. One preclinical study created a diabetic human skin equivalent (DHSE) that was made of fibroblasts and keratinocytes extracted from diabetic patients’ own skin, cultures in vitro, and incorporated on top of synthetic membranes.55 The DHSE was applied and electrical stimulation (ES) therapy was used, and this combination led to increased wound healing.55 This technique could potentially be used as an autologous graft to cover DFU.55
There are various pipeline products currently undergoing clinical trials, one of them being the ReGenerCelL Autologous Cell Harvesting Device.56 This device entails using a small portion of the patient’s own skin to produce Spray-On-Skin Cells, and this suspension has cells necessary for regenerating the outer layer of skin.57 It is currently approved for burn wounds, but is undergoing clinical trials to determine its efficacy in treating DFU.56 ExpressGraft-C9T1 Skin Tissue is the first genetically engineered skin substitute that generates higher levels of cathelicidin.58–60 Cathelicidin has antimicrobial properties and promotes angiogenesis and epithelialization, so this option could lead to increased rates of healing.60 Another potential product encapsulates ADSCs onto a fibrin gel, which then allows the ADSCs to migrate out of the gel and onto the wound bed, where they will differentiate into many cells that lead to enhanced healing.61,62
Cell2Cure uses adipose-derived stem cells to try and create ready-made products that are easy to use.63,64 When ASCs interact with diseased tissue, this results in secretion of many factors that can reduce inflammation, fibrosis and scar formation, as well as promote angiogenesis.64 TTAXO1 is the current name for the cryopreserved human amniotic membrane and umbilical cord (AM/UC) matrix.65,66 This product is unique in that it can be used even in cases with exposed bone and severe osteomyelitis, due to the anti-inflammatory properties of the umbilical cord and other active matrix components.67,68
PalinGen Flow is a human amniotic tissue allograft that is similar to Amnio Technolgy’s other products, but this comes in liquid form, allowing for subcutaneous injection.69 Kerecis Dura is an innovative product that utilizes intact fish skin to create cost-effective grafts that have no risk of disease transmission and minimal risk of inflammation.70,71 Another advantage to this product is that it is very porous, which promotes cell migration, and it will not leave any foreign materials when it gets absorbed.70,71 No other biologic scaffold retains lipids, but the current acellular fish scaffolds from Kerecis retain their omega 3 fatty acids, which helps reduce the inflammatory response.71 A comparison of the current products with the pipeline products are demonstrated in Table 5.72
|
Product |
Single use |
Long |
No |
Costeffective |
Highly Antiinflammatory |
Lack of Stem cells |
Very low Risk of |
Applicable for cases with osteomyelitis |
|
|
|
shelf life |
freezer required |
|
|
infection transmission |
||
|
Apligraf |
✔ |
✘ |
✘ |
✘ |
✘ |
✔ |
✘ |
✘ |
|
Dermagraft |
✘ |
✘ |
✘ |
✘ |
✘ |
✔ |
✘ |
✘ |
|
Theraskin |
✘ |
✔ |
✘ |
✘ |
✔ |
✔ |
✘ |
✔ |
|
Oasis |
✘ |
✔ |
✔ |
✘ |
✘ |
✔ |
✘ |
✘ |
|
DermAcell |
✔ |
✔ |
✔ |
✔ |
✘ |
✔ |
✘ |
✘ |
|
Epifix |
✘ |
✔ |
✔ |
✘ |
✘ |
✘ |
✘ |
✘ |
|
Integra DRT |
✔ |
✔ |
✔ |
✘ |
✘ |
✔ |
✘ |
✘ |
|
Grafix |
✘ |
✔ |
✘ |
✘ |
✔ |
✘ |
✘ |
✘ |
|
AmnioBand |
✘ |
✔ |
✔ |
✘ |
✔ |
✘ |
✘ |
✘ |
|
ADSCs, fibrin |
- |
- |
✘ |
- |
✘ |
✘ |
✘ |
✘ |
|
Cell2Cure |
- |
✔ |
✘ |
✘ |
✘ |
✘ |
✘ |
✘ |
|
TTAX01 |
- |
- |
✘ |
- |
✔ |
✘ |
✘ |
✔ |
|
Kerecis Dura |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✘ |
Table 5 Product and uses
DFUs present a growing concern due to their impact on quality of life and economic burdens at all levels, from the individual to global expenses. However, there are promising treatments available for effectively healing DFUs across a range of severity, from mild to complex cases involving significant exposure of the underlying anatomy, as well as infection. Researchers and companies worldwide are actively exploring various solutions, leading to an ever-growing diversity of proposed treatments. While some advanced therapies are quite expensive, ongoing studies are dedicated to developing efficient, cost-effective alternatives that could be accessible for all individuals, regardless of socioeconomic status. The urgency to combat DFU-related morbidity and mortality has sparked a wave of progress, and DFU treatment has the potential to become revolutionized and significantly improve outcomes worldwide.
None.
There are no conflicts of interest presented or declared by the authors in this research.
None.

©2023 Lina, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.