Journal of
eISSN: 2572-8466


Research Article Volume 5 Issue 5
1Department of Food Engineering and Technology, Institute of Chemical Technology, India
2DBT-ICT Centre for Energy Biosciences, Institute of Chemical Technology, India
Correspondence: Annamma A Odaneth, DBT-ICT Centre for Energy Biosciences, Institute of Chemical Technology, Nathalal Parekh Marg, Mumbai 400019, India, Tel 91-22-33612312
Received: July 26, 2018 | Published: October 23, 2018
Citation: Shaikh AE, Pawar A, Parmar H, et al. Conjugated Linoleic Acid production by Lactic Acid Bacteria: A Bio-transformation study in media with oil hydrolysates. J Appl Biotechnol Bioeng. 2018;5(5)321-327. DOI: 10.15406/jabb.2018.05.00158
The unique ability of lactic acid bacteria (LAB) to convert unsaturated fatty acids to saturated fatty acids via the production of unsaturated intermediates is known as bio-hydrogenation, which is responsible for the conversion or the bio-transformation of unsaturated fatty acids like linoleic acid to saturated fatty acids like stearic acid via the intermediate compounds such as conjugated linoleic acid (CLA). Realizing the health benefits of CLA, lactic acid bacteria were isolated from curd in order to study their ability of CLA production. Initially, four bacterial strains isolated from curd and designated as 1s, 13s, 15s, and 17s were screened and found to be gram-positive, catalase negative and acid producers. Out of these four strains, 1s and 13s showed rapid adaptation to the change in the substrate from glucose to linseed oil hydrolysate. The fermentation study conducted in shake flask mode revealed that the maximum concentration of trans-10, cis-12 CLA produced in medium enriched with 0.4% (w/v) castor oil hydrolysate (mCOH) by isolate 1s was 620 mg·L-1 at 4h while that produced by isolate 13s in mCOH was 430mg·L-1 at 8h as assessed by gas chromatography (GC) performed using a flame ionization detector (FID). Thus, the study proved the ability of LAB to synthesize CLA.
Furthermore, in order to test the feasibility of CLA production on a large scale, an additional study was conducted using Lactobacillus delbreukii NCIM 2025 in a continuously stirred tank reactor in both batch and continuous modes using linseed oil hydrolysate as the fatty acid substrate. The maximum concentration of CLA produced by Lactobacillus delbreukii NCIM 2025 in medium enriched with 0.4% linseed oil hydrolysate (mLOH) was 380µg·L-1 at 6 h in batch mode and 590 µg·L-1 at 1h in continuous mode as analyzed using high-performance liquid chromatography (HPLC) performed using an ultraviolet (UV) detector at 233nm. Thus, the study also reveals that there is a vast scope for improvement in the scale-up of CLA production from the laboratory scale to a commercial scale.
Keywords: conjugated linoleic acid, lactic acid bacteria, bio-transformation, linseed oil hydrolysate, castor oil hydrolysate
Conjugated linoleic acid (CLA) is a general term used for the positional and geometric isomers of linoleic acid (C18:2) which have double bonds in conjugation. Different isomers of CLA have been identified as potential therapeutic molecules and it has been reported that CLA has anti-carcinogenic effects in animal models1 and also anti-inflammatory,2 anti-diabetic3,4 anti-obesity5 and anti-atherogenic6 effects. Also, properties such as anti-hyper blood pressure, anti-oxidant, and cholesterol-reduction have been reported.71 The cis-9, trans-11 isomer of CLA is responsible for the anti-carcinogenic activity8,9 while the trans-10, cis-12 isomer is responsible for weight control.5
The primary natural food sources of CLA come from the ruminants, be it the meat, milk or dairy products originating from them. However, some vegetables and some sea-foods have also been reported to contain CLA.10,11 However, the CLA levels in these natural sources are insufficient to confer the essential health benefits. Therefore, CLA is synthesized by using chemical processes and microbial fermentation. However, the chemical synthesis methods produce a mixture of many positional isomers of CLA and thus, fail to differentiate between the biological activities of the isomers that they produce; some of these isomers may be biologically active while some may be relatively inactive.12 On the other hand, CLA production using microbial fermentations produce only the biologically active isomers of CLA. This selective production of only those CLA isomers which are bioactive enables the fermentative production of CLA to be advantageous over their production through chemical synthesis.
It is widely known that unsaturated fatty acids are prone to oxidation and generate intermediates such as free radicals, which are harmful to a living cell. Organisms like lactic acid bacteria (LAB) are no exception to this susceptibility to the deteriorative effects caused by the oxidation of unsaturated fatty acids. In order to avoid these effects, LAB metabolize the unsaturated fatty acids to saturated fatty acids and thereby this process is known as bio-hydrogenation.13 When the unsaturated fatty acids such as linoleic acid (C18:2) are present in the environment, the bio-hydrogenation involves the conversion of linoleic acid (C18:2) to stearic acid (C18:0). However, this conversion involves different unsaturated intermediates, conjugated linoleic acids being among them.14 Thus, LAB possess a unique ability for the conversion or the bio-transformation of the unsaturated fatty acids to conjugated linoleic acid as an intermediate of the bio-hydrogenation pathway.
Realizing the health benefits of CLA, the need to produce it artificially for potential supplementation in the human diet, and the unique ability of LAB to synthesize it, the present study attempts to explore the ability of LAB to synthesize CLA in fermentation matrices, which were enriched with fatty acid substrates such as oil hydrolysates. The study includes the isolation of lactic acid bacteria from curd, screening them for various characteristics including metabolic potential, making them toadapt to fatty acids as the carbon source instead of glucose, and using the rapidly adapting strains for studying fermentative bio-transformation of fatty acids in castor oil hydrolysate to CLA through shake flask experiments. Moreover, in order to study the feasibility of the commercialization of CLA production using LAB, Lactobacillus delbruekii NCIM 2025 was used for studying fermentative bio-transformation of fatty acids in linseed oil hydrolysate to CLA in continuously stirred tank reactor (CSTR) operating in batch and continuous modes. The CLA synthesized using LAB can be utilized after purification from the fermentation media for applications in foods, pharmaceuticals, and nutraceuticals and the present study confers its contribution towards the CLA synthesis and attempts to highlight the need for scale-up, which is essential to facilitate its commercialization.
Materials
Curd used for the isolation of lactic acid bacteria was obtained from local dairy shop and stored at 4°C until use. Cold pressed linseed oil (Health First®) was obtained from local market and castor oil was given as a gift by Acme Synthetic Chemicals Ltd., Mumbai, India. These oils were converted to their respective hydrolysates using immobilized lipase-based process optimized by Vadgama.15 The strain, Lactobacillus delbruekii NCIM 2025 was obtained from National Collection of Industrial Microorganisms, National Chemical Laboratory, Pune, India. The following chemicals were obtained from Hi-Media, Mumbai, India: de Man Rogosa Sharpe (MRS) medium, agar, crystal violet, Gram’s iodine, safranin, peptone, beef extract, yeast extract, glucose, Tween-80, anhydrous manganese sulphate, anhydrous sodium acetate, ammonium sulphate, triammonium citrate, anhydrous potassium phosphate dibasic, magnesium sulphate heptahydrate, manganese sulphate monohydrate and boron trifluoride methanol complex (14% in methanol). Analytical grade hexane, absolute ethyl alcohol, acetonitrile and hydrogen peroxide were obtained from SD Fine Chemicals Pvt Ltd, Boisor, India whereas bromocresol purple (BCP) indicator (AR grade) and ortho-phosphoric acid (AR grade) were obtained from Alpha Chemika, Mumbai and SD Fine Chemicals Pvt Ltd, Boisor, India respectively. De-ionized (Milli Q Water System, Milipore, India) water was used for all media preparations and analyses. The CLA used in chromatographic analyses as a standard was obtained from Sigma Aldrich, USA.
Methods
Fermentative production of conjugated linoleic acid in medium enriched with castor oil hydrolysate
This part of the study is an extension to the work carried out by Warke et al.16 and the methods of isolation, adaptation, screening and analysis of fatty acids are thereby borrowed with some modifications and are briefly described below.
Isolation of lactic acid bacteria
The curd sample was serially diluted in saline and this was followed by spread plate technique on de Man Rogosa Sharpe (MRS) with Bromocresol purple (BCP) agar plates having the composition: 10g·L-1 peptone, 10 g·L-1 beef extract, 5g·L-1 yeast extract, 20g·L-1 glucose, 1g·L-1 Tween 80, 5g·L-1 sodium acetate, 2g·L-1 ammonium citrate, 2g·L-1 K2HPO4, 0.1 g·L-1 MgSO4.7H2O, 0.05g·L-1 MnSO4.H20, 0.2 g·L-1 bromocresol purple (BCP) and 20 g·L-1 agar with the final pH of the medium close to 7.0. These plates were then covered with 10g·L-1 agar to maintain microaerophilic condition and incubated at 28±2°C for a period of 48h in an incubator shaker (Remi Orbital Shaker, India) at 200rpm. The colonies showing yellow zones around them were picked and inoculated in 10mL MRS-BCP broth and incubated at 28±2°C for 48h at 200rpm. After the incubation period, the colonies that showed an intense yellow coloration in the broth were tested for gram nature and catalase activity. Gram nature was tested using gram staining technique while for the catalase test, a 10µL aliquot of cultured broth was placed in a drop of 3% hydrogen peroxide solution. The cultures which were found to be gram-positive and catalase negative were purified on fresh MRS-BCP agar plates. The purified isolates were then maintained on MRS agar slants at 4°C.
Adaptation of isolates to utilize fatty acids
The isolated lactic acid bacteria were gradually adapted to utilize fatty acids as the carbon source by corresponding replacement of glucose with linseed oil hydrolysate in the MRS-BCP broth. The isolates were initially inoculated in 5mL MRS-BCP broth having 20g·L-1 glucose and no linseed oil hydrolysate and incubated at 28±2°C for 48h in an incubator shaker (Remi Orbital Shaker, India) at 200rpm. The colonies showing yellow coloration in the broth were then inoculated from this broth into 5mL MRS-BCP broth having 10g·L-1 each of glucose and linseed oil hydrolysate and incubated at 28±2°C for 48 h at 200 rpm. The colonies that showed intense yellow coloration in this broth were finally inoculated in MRS-BCP broth having 20g·L-1 linseed oil hydrolysate and no glucose and incubated at 28±2°C for 24 h at 200rpm. The isolates which showed intense yellow coloration in this broth were then used for CLA production.
Conjugated linoleic acid production using adapted isolates
The isolates selected on the basis of rapid adaptation to utilize fatty acids for growth were used for the production of CLA. For this, the adapted isolates were inoculated in 50mL MRS-BCP broth having 20g·L-1 glucose and 0.6 g·L-1 linseed oil hydrolysate for activation and incubated at 37°C for 12h in an incubator shaker (Remi Orbital Shaker, India) at 200rpm. After the activation, approximately 0.5g of washed biomass obtained by centrifugation at 12,857g for 10min in Eppendorf 5810R centrifuge was inoculated in 25mL production medium enriched with castor oil hydrolysate (mCOH) [composition (g·L-1): 0.05g MnSO4, 5g sodium acetate, 2g ammonium sulphate, 5g yeast extract, 4g castor oil hydrolysate and 0.2g Tween-80] and the bio-transformation was monitored for 12 h over regular intervals of 4 h at 37 ºC at 200rpm.
Gas chromatographic analysis of fatty acids
After the incubation period, the cells were removed by centrifugation at 12,857g for 10min and the supernatant was used to extract fatty acids using hexane as the solvent. The solvent was separated using vacuum distillation (Heidolph Laborota 4002 control) and the fatty acids were esterified to Fatty Acid Methyl Esters (FAME) using Boron Trifluoride – Methanol solvent system.17 Finally, the FAME samples were analyzed using Gas Chromatography – Flame Ionization Detector (Agilent Technologies Canada, Kirkland, QC, Canada) using high cyanopropyl containing polysiloxane HP88 column (100mx0.25mmx0.25µm).The carrier gas used was nitrogen with a flow rate of 0.5mL·min-1. The injection volume used was 2µL with split ratio of 5:1. The temperatures of the injector and the detector were 250°C each. The oven was operated at an initial temperature of 80°C followed by the following process of temperature rise: increase to 150°C at a ramp rate of 20°C·min-1; holding at 150°C for 1min; increase to 200°C at a ramp rate of 5°C·min-1; holding at 200°C for 10 min; increase to 230°C at a ramp rate of 2°C·min-1; holding at 230°C for 10 min. Also, standard CLA (Sigma Aldrich, USA) was used to calculate concentration of CLA in the samples.
Fermentative production of CLA in medium enriched with linseed oil hydrolysate
This part of the study constituted optimization of the concentration of linseed oil hydrolysate in production medium enriched with linseed oil hydrolysate (mLOH) by carrying out fermentation in shake flask mode using Lactobacillus delbruekii NCIM 2025. Further, this optimized medium was used to study production of CLA by L. delbruekii NCIM 2025 in continuously stirred tank reactors (CSTR) in batch and continuous modes.
Optimization of linseed oil hydrolysate concentration in production medium
A loopful of the culture (0.1mL) of Lactobacillus delbruekii NCIM 2025 was inoculated in MRS broth and incubated at 30°C for 24 h. After 24 h of incubation at 30°C, a 2.5mL aliquot of this seed culture was inoculated in 50mL production medium enriched with varying concentrations of linseed oil hydrolysate and incubated at 30°C for 24 h in a rotary orbital shaker (Remi Orbital Shaker, India) at 200rpm. The composition of the production medium was as follows: 5 g·L-1 yeast extract, 5 g·L-1 sodium acetate, 2 g·L-1 ammonium sulphate; 0.05 g·L-1 tween 80 and linseed oil hydrolysate at concentrations ranging from 2 g·L-1 to 10g·L-1. The initial pH of the media was about 7.0. After 24h the broths were centrifuged at 7,800g for 15 min in a Sigma 6-16KS centrifuge machine to separate the cells from the broth and the supernatant was subjected to treatment with 100mL hexane for extraction of fatty acids. The hexane layer was further subjected to evaporation and consequently the fatty acids were analyzed as per the protocol described in the section on high performance liquid chromatographic analysis of fatty acids.
CLA production by Lactobacillus delbruekii NCIM 2025 in continuously stirred tank reactor in batch mode
The batch fermentation was carried out in a fermentor (3L capacity, Bio-Age, Mohali, India) with a working volume of 2 L and agitation speed of 200 rpm. The composition of the production medium used was as follows: 5g·L-1 yeast extract, 5g·L-1 sodium acetate, 2g·L-1 ammonium sulphate; 0.05g·L-1 tween 80 and 4g·L-1 linseed oil hydrolysate. The inoculum used was a 100mL MRS broth containing 24 h seed culture of L. delbrueckii NCIM 2025 grown at 30°C (i.e. 5% v/v inoculum). The pH of the medium was maintained at pH 7.0 using 5% ammonia solution and the temperature was maintained at 30±2°C during the course of the fermentation. A 2mL sample was withdrawn from the fermentation broth at intervals of 1h for 6h and at 12h and 18h. The supernatant obtained after centrifugation of the samples at 7,800 g for 15 min in a Sigma 6-16KS centrifuge machine was then subjected to 10mL hexane treatment followed by evaporation of the hexane layer and subsequent analysis by high performance liquid chromatography as described in the section on high performance liquid chromatographic analysis of fatty acids.
CLA production by Lactobacillus delbruekii NCIM 2025 in continuously stirred tank reactor in continuous mode
The continuous mode of CLA production was studied using lineup of Sartorius Stedim Biostat B Plus fermentor and hollow fiber membrane of 0.2 micron (GE Healthcare Bio-Sciences Corp, USA). Initially, the fermentor with a working volume of 1 L of MRS broth was inoculated with 5% v/v of a 24h seed culture of L. delbruekii NCIM 2025 grown in MRS broth and kept at temperature of 30°C with agitation speed of 200rpm. After 24 hours of growth time, the hollow fiber microfiltration membrane of size 0.2 micron was connected to the fermentor with a 1mL·min-1 inlet flow rate of production medium (composition: 5g·L-1 yeast extract, 5 g·L-1 sodium acetate, 2g·L-1 ammonium sulphate; 0.05 g·L-1 tween 80 and 4g·L-1 linseed oil hydrolysate). The production medium at 1mL·min-1 flowrate was passed through the fermentor to gradually replace the MRS brothand the pH of the medium was maintained at 7.0 using 5% ammonia solution. Then, 2mL samples from permeate were withdrawn at regular intervals of time; centrifuged at 7,800g for 15 min in a Sigma 6-16KS centrifuge machine and the supernatent was treated with 10mL hexane for extraction of fatty acids. The hexane layer was then evaporated and the fatty acids were analyzed using HPLC analysis using procedure described in the section on high performance liquid chromatographic analysis of fatty acids.
High performance liquid chromatographic analysis of fatty acids
The supernatant obtained after centrifugation of the fermentation broth was subjected to hexane extraction. This extract obtained in hexane was evaporated at 70°C under a pressure of 200 bar in a Heidolph Laborota 4002 rotary evaporator. The sample left was suitably diluted in tertiary butanol and filtered through a 0.2 micron nylon syringe filter (Axiva Sichem Pvt Ltd, Delhi, India). A 10μL aliquot was injected into an Agilent Zorbax C-18 reverse phase HPLC column (4.6mm internal diameter, 250mm length and 5μm particle size) at 30°C. The mobile phase consisted of acetonitrile (ACN) (solvent A) and 30 mM phosphoric acid in water (solvent B) with a flow rate of 1mL·min-1. The gradient started at 80% A followed by a linear increase to 90% A in 15min. One hundred per cent A was then held for 20 min followed by return to initial condition in next 5 min. The detection of CLA was carried out at 233 nm by UV detector. The concentration of the sample was determined from the area under the peak for standard conjugated linoleic acid (Sigma Aldrich, USA).
Fermentative production of conjugated linoleic acid in medium enriched with castor oil hydrolysate
Four distinct colonies, designated as 1s, 13s, 15s and 17s, showed yellow zones around them on MRS-BCP agar plates after incubating for 48 h at 28±2°C. They were stained violet and appeared rod-shaped in gram staining and developed no effervescence with hydrogen peroxide. Thus, all the four isolates were identified as belonging to the genus Lactobacillus based on their acid producing ability, gram positive nature, rod-shaped morphology and absence of catalase.Out of the four isolates, 1s and 13s showed more intense yellow coloration in the MRS-BCP broth (Figure 1) with 2% glucose and no linseed oil hydrolysate than that shown by isolates 15s and 17s after 48 h of incubation at 28±2°C. This indicated that isolates 1s and 13s were more vigorous acid producers than isolates 15s and 17s. Hence, the adapted isolates 1s and 13s were subjected to adaptation in MRS-BCP broth with 1% each of glucose and linseed oil hydrolysate. After 48 h of incubation at 28±2°C, both the isolates showed intense yellow coloration in the broth (Figure 1) indicating successful adaptation to the change in substrate type and concentration. Finally, upon inoculating isolates 1s and 13s in MRS-BCP broth with 2% linseed oil hydrolysate and with no glucose, yellow coloration was observed in the broth (Figure 1) after 24 h of incubation at 28±2°C revealing that the organisms had successfully adapted to grow on fatty acids as the substrate. These results have also been tabulated in Table 1. Upon monitoring of the fermentation in the production medium (mCOH) by the adapted isolates 1s and 13s using gas chromatography, it was revealed that both the isolates produced trans-10, cis-12 conjugated linoleic acid. Isolate 1s produced 0.62 g·L-1 of trans-10, cis-12 CLA at 4 h of fermentation, which later began to decline (Table 2). On the other hand, isolate 13s gradually produced 0.13 g·L-1 of trans-10, cis-12 CLA at 4h which increased to 0.43g·L-1 at 8h of fermentation and then declined to 0.04 g·L-1 at 12h.
Isolate |
MRS-BCP |
MRS-BCP |
MRS-BCP |
1s |
++ |
++ |
+++ |
13s |
++ |
++ |
+++ |
15s |
+ |
N.A.* |
N.A.* |
17s |
+ |
N.A.* |
N.A.* |
Table 1Effect of change in substrate for adaptation on growth of isolates indicated by intensity of yellow coloration in MRS-BCP broth (+ = Brown-yellow; ++ = Orange-yellow; +++ = Yellow)
*N.A., Not Applicable
Incubation time (h) |
Concentration of trans-10, cis-12 CLA (mg per 100 mL mCOH) |
|
Isolate 1s |
Isolate 13s |
|
4 |
61.81 |
12.65 |
8 |
0.57 |
43.05 |
12 |
N.D.* |
4.47 |
Table 2 CLA production by isolates 1s and 13s during fermentation in medium enriched with castor oil hydrolysate over 12 h period at 37°C
*N.D., Not Detected
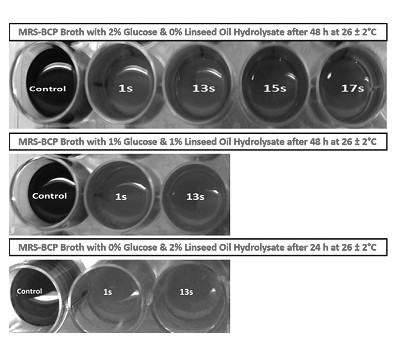
Figure 1 Effect of change in substrate for adaptation on growth of isolates indicated by intensity of yellow coloration in MRS-BCP broth.
Fermentative production of CLA in medium enriched with linseed oil hydrolysate
It was found that Lactobacillus delbruekii NCIM 2025 produced conjugated linoleic acid in the production medium at all concentrations of linseed oil hydrolysate used which were 2, 4, 6, 8, 10 and 12 g·L-1. However, the maximum concentration of CLA, which was 23 µg·L-1, was found to be produced in the medium with 4 g·L-1 linseed oil hydrolysate. Upon increase in the concentrations of the fatty acid substrate, which is linseed oil hydrolysate containing linoleic acid, the CLA production by L. delbruekii NCIM 2025 increased. However, this increase in production was only observed till the concentration of linseed oil hydrolysateused was 0.4% (w/v). Later, the CLA production decreased as the concentrations increased beyond 0.4% (w/v). Thus, the production medium with 0.4% (w/v) linseed oil hydrolysate was selected for CLA production by L. delbruekii NCIM 2025 in continuously stirred tank reactor (CSTR) in batch and continuous modes.
Lactobacillus delbruekii NCIM 2025 was found to successfully produce CLA at linseed oil concentration of 0.4% (w/v) during the course of fermentation in CSTR in a batch mode at 30°C and 200rpm. The concentration of CLA produced was found to increase upto 6 h and later declined. The maximum concentration of CLA produced by L. delbruekii NCIM 2025 was found to be 380µg·L-1 at 6 h after inoculation. The maximum concentration of CLA produced by L. delbruekii in CSTR in batch mode (380µg·L-1) was found to be higher than that produced by it in shake flask study (23µg·L-1) at the same linseed oil hydrolysate concentration of 0.4% (w/v). Also, L. delbruekii NCIM 2025 produced 360 µg·L-1 CLA in 6 h after inoculation as compared to production of 23µg·L-1 CLA in 24 h after inoculation in shake flask study whereby the substrate concentration and inoculum concentration were same in both the cases i.e. 0.4% linseed oil hydrolysate and 5% v/v of a 24 h seed culture grown in MRS broth. This shows that the productivity of CLA by L. delbruekii NCIM 2025in batch mode in a CSTR was more than that in shake flask mode.
The CLA production by L. delbruekii NCIM 2025 in CSTR operated in a continuous mode was studied for a period of 3 h and it was found that the CLA produced reached a maximum concentration of 590 µg·L-1 after 1 h and later decreased and remained approximately constant. The maximum CLA production by L. delbruekii NCIM 2025 in continuous mode, which was 590 µg·L-1, was found to be higher than the maximum CLA produced by it in batch mode which was 380 µg·L-1. Also the CLA productivity of L. delbruekii NCIM 2025 was higher in continuous mode than in the batch mode as higher production of CLA in the continuous mode was found to take place in lesser time. However, the CLA isomers produced were not differentiated in this part of the study as the analysis of the fatty acids was done using High Performance Liquid Chromatography.
Fermentative production of conjugated linoleic acid in medium enriched with castor oil hydrolysate
Milk is one of the common food substances that harbors lactic acid bacteria, which are also resposible for milk-derived products like curd being a rich source of these bacteria. The spread plate technique led to the successful isolation of four distinct colonies of lactic acid bacteria from curd, which were identified as Lactobacillus sp. on the basis of acid production revealed by the yellow coloration in the MRS-BCP broth and their gram-positive and catalase-negative nature along with their rod shaped morphology. The intensity of yellow coloration in MRS-BCP broth is proportional to the amount of acid produced by the lactic acid bacteria since BCP dye changes its color to yellow in acidic conditions. Based on this, the isolates 1s and 13s showing more intense yellow coloration than isolates 15s and 17s were adapted to utilize fatty acids. Both the isolates adapted to the change in substrate type and concentration from simple carbohydrate to fatty acids as the sole carbon source as indicated by the yellow coloration in the MRS-BCP broths substituted with linseed oil hydrolysate instead of glucose.
The fermentation studies conducted using these isolates in medium enriched with castor oil hydrolysate (mCOH) showed that the CLA concentration in the mediumincreased initially and later declined. This is because the lactic acid bacteria produce CLA as an intermediate of the biohydrogenation pathway, and hence the CLA concentration in the medium increases initially due to the biotransformation of ricinoleic acid present inthe castor oil hydrolysate to CLA18 and then decreases as the biohydrogenation proceeds further, in which the CLA is ultimately converted to saturated fatty acids by the bacteria.19,20 Furthermore, the difference between the CLA producing abilities of the two Lactobacillus species and the time period after which the CLA concentration produced reached a maximum is due to differences in their response to utilization of substrate, which were fatty acids. It can also be attributed to the differences in the metabolic rates between the two isolates 1s and 13s, which is indicative of species-dependent CLA production by the lactic acid bacteria. Moreover, itis clear from the GC chromatograms (Figure 2) that both the isolates, 1s and 13s produced only the biologically beneficial isomer of CLA, which is trans-10, cis-12 CLA, which has the potential for weight control.5 Thus, the maximum concentration of this isomer produced by strains 1s and 13s were 0.62g·L-1 after 4 h and 0.43g·L-1 after 8 h respectively.
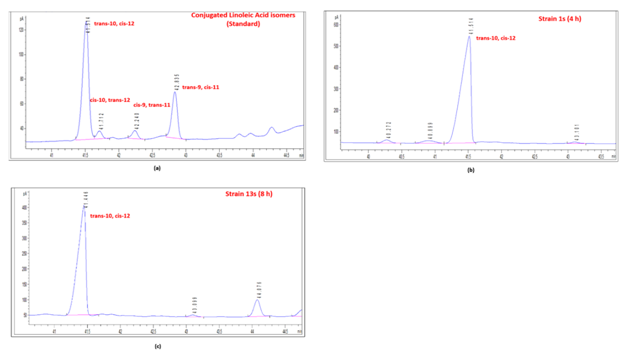
Figure 2 GC Chromatograms. a) Standard CLA isomers, b) CLA production at 4 h by isolate 1s, c) CLA production at 8 h by isolate 13s.
Similar results were reported by Warke et al.16 for Lactobacillus delbruekii NCIM 2025 which produced 0.69g·L-1 of total CLA, out of which 0.59 g·L-1 was cis-9, trans-11 isomer, while the rest was accounted by trans-10, cis-12 and trans-9, cis-11 isomers after 6 h of incubation. They also reported Leuconostoc lactis and Wesissella viridescenses to produce 0.1 g·L-1CLA, which was accounted by the trans-10, cis-12 and trans-9, cis-11 isomers. These findings were reported for the initial pH of the fermentation medium, incubation temperature, size of microbial inoculum, substrate concentration and activation method, which were same as those used in the present study. Thus, taking these into consideration, it can be stated that under similar parameters of pH, temperature, microbial inoculum size, substrate concentration and activation method, the amount of total CLA produced by lactic acid bacteria is genus-dependent while the distribution of different isomers accounting to the cumulative amount of CLA produced is further species-dependent as well. This may be due to the similar metabolic potentials of lactic acid bacteria belonging to the same genus while varying selectivities of the enzymes produced by them for the bio-transformation of ricinoleic acid (a major component in castor oil hydrolysate) to CLA due to the variations in the three-dimensional conformation of their respective enzymes.
Ando et al.21 reported Lactobacillus plantarum JCM 1551 to produce 2.7g·L-1 of CLA, which was accounted by the cis-9, trans-11 and trans-9, trans-11 isomers after 99 h of incubation. However, these findings for bacteria belonging to the genus Lactobacillus are different from those reported in the present study as they were reported at fermentation parameters different than those used in the present study, such as initial pH of 6.0, microbial inoculum of 12% w/v, substrate as 5g·L-1 castor oil added along with lipase M “Amano” 10, incubation in medium with 0.13% α-linoleic acid and use of Lubrol PX in reaction mixture for activation except incubation temperature which was same as 37°C. This indicates that there is a great scope for improvement in CLA production through the optimization of reaction conditions and fermentation medium composition using the lactic acid bacteria isolated from curd.
Fermentative production of CLA in medium enriched with linseed oil hydrolysate
Lactic acid bacteria produce conjugated linoleic acid as an intermediate in the biohydrogenation pathway as a detoxification mechanism to convert unsaturated fatty acids to saturated fatty acids. Linoleate isomerase is an enzyme involved in the biohydrogenation of linoleic acid to CLA. Lactobacillus delbruekii NCIM 2025 produced CLA by converting the linoleic acid present in the linseed oil hydrolysate via this biohydrogenation pathway.22 However,the CLA production was found to decrease beyond linseed oil hydrolysate concentration of 0.4% (w/v)in the shake flask study due to the inhibition of the activity of linoleate isomerase at substrate concentration above 0.4% (w/v). Thus, it can be inferred that an optimum concentration of linseed oil hydrolysate in the production medium is 0.4% (w/v).
CLA production using various species of the genus Lactobacillus in MRS broth with added linoleic acid has been reported and it has been found that various strains have shown varying abilities of CLA production.23,24 Among the L. acidophilus strains, L. acidophilus ADH was found to produce 630 mg·L-1 (Xu et al. 2008) while lower production was reported for the strains L. acidophilus Ki and L. acidophilus LAC1 as 8.57 mg·L-1 and 3.89 mg·L-1 respectively at 0.1% linoleic acid concentration after 24 h of incubation at 37°C (Rodríguez-Alcalá et al. 2011). Comparatively, L. delbruekii NCIM 2025 was found to produce lesser CLA than the Lactobacillus strains reported in the literature (Xu et al. 2008; Rodríguez-Alcalá et al. 2011). These variations in the amount of CLA produced occur due to different tolerance limits of the strains to linoleic acid in the medium beyond which the linoleate isomerase enzyme, which is responsible for the bio-transformation of linoleic acid to conjugated linoleic acid is inhibited. Also the incubation temperature was different in the studies reported (Xu et al. 2008; Rodríguez-Alcalá et al. 2011) which was 37°C than that used in the present study which is 30°C, which may have accounted for the differences in the biotransformation activity of linoleate isomerase.
However, the increase in the CLA concentration when the experimentationwas carried out using production medium in a continuously stirred tank reactor in batch mode can be attributed to the increased homegeneity of the nutrients in the medium and also the increased dispersion of the immiscible phases of oil hydrolysate and water in the medium due to the continuous mechanical agitation used in a CSTR. Also this agitation leads to a uniform distribution of temperature and pH conditions within the medium leading to conducive environment for the growth of cells in all parts of the fermentor. It also keeps the microbial biomass suspended in the medium for increased utilization and conversion of the substrate to CLA.
Further, the enhanced productivity of CLA in continuous mode in CSTR indicates that the efficiency of the biotransformation increased in the continuous mode as compared to the batch mode. This can be attributed to the continuous removal of the product from the system along with the continuous supply of fresh medium containing nutrients preventing reaction inhibition. Thereby, after a certain period of time, the CLA production in the continuous mode reached approximately a steady state while the CLA production in batch mode declined in the later stages of the fermentation (Figure 3).Thus, both the batch and continuous modes of fermentation in continuously stirred tank reactor were successfully able to improve the productivity of conjugated linoleic acid by L. delbruekii NCIM 2025 as compared to the shake flask experiments and these strategies could be employed for the industrial production of CLA using L. delbruekii NCIM 2025.
Lactic acid bacteria prove to be promising organisms for the production of CLA through fermentation in fatty acid rich matrices like media with oil hydrolysates. Different strains have varying abilities of CLA production due to differences in their metabolic potential. Also, CLA production using micro-organisms leads to the formation of only the biologically active isomers. However, the type of biologically active isomer produced by different organisms is different due to the differences in the enzymatic conformations leading to varied specificities. It was also proved that CLA production was enhanced in continuous mode of operation as compared to the batch mode in a CSTR which highlights the opportunities that can be explored in order to produce CLA on a commercial scale. It also highlights that there is an extensive scope for improvement in the yields of CLA produced by organisms with lower metabolic potential. This can be achievedthrough optimization of thecomposition of fermentation medium, operating conditions of the reactor and also through the optimum use of biomass concentration for the bio-transformation. Further advancements like genetic manipulations of these bacteria for over-production of CLA and also the use of immobilized cells for improved conversions in continuous bioreactors may lead to enhanced productivity of CLA. Such efforts may eventually lead to the development of technology with reduced cost and easy commercialization for the microbial production of conjugated linoleic acids.
This article does not contain any studies with human participants or animals performed by any of the authors
The first author is grateful to Dr. Lakshmi Narayanan for his immense technical support.
Compliance with ethical standards
This work received funding from the Department of Biotechnology, Ministry of Science and Technology, Government of India.
Anas Shaikh declares that he has no conflicts of interest. Ankita Pawar declares that she has no conflicts of interest. Hemant Parmar declares that he has no conflicts of interest. Rajeshkumar Vadgama declares that he has no conflicts of interest. Annamma Odaneth declares that she has no conflicts of interest. Arvind Lali declares that he has no conflicts of interest.

©2018 Shaikh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.