Journal of
eISSN: 2572-8466


Research Article Volume 10 Issue 4
1Post-Graduation Program in Natural Resources, State University of Mato Grosso do Sul, Brazil
2Department of Chemistry, State University of Maringá, Brazil
Correspondence: Viviane Mallmann, Universidade Estadual de Mato Grosso do Sul, Programa de Pós-Graduação em Recursos Naturais, Dourados, MS, Brasil
Received: July 12, 2023 | Published: September 15, 2023
Citation: Mallmann V, Aragão LWR, Bueno D, et al. Chemical and antibacterial evaluation of the essential oil from the leaves, petals and calyx of Calea phyllolepis Baker in Brazil. J Appl Biotechnol Bioeng. 2023;10(4):120-127. DOI: 10.15406/jabb.2023.10.00337
In the face of the complex context of resistance of human pathogenic bacteria, essential oils act in a synergistic way preventing the bacterial mechanism to create resistance. This article brings the chemical identification of the essential oils of the leaves, petals, and calyx of C. phyllolepis and the evaluation of these in the control of S. aureus and E. coli (Minimum Inhibitory Concentration (MIC) and Disc Diffusion). Thirteen compounds were identified in the EOs, being five monoterpenes and eight sesquiterpenes. Sabinene, (-)-α-Pinene and ρ-Cymene presented with high concentrations in all the evaluated parts. The minimum concentrations to effectively inhibit the development of bacteria varied between 0.3% to 4.0% against S. aureus and 0.3% to 8.0% for E. coli in the colorimetric assay. The most effective action against the bacteria studied were found for petal and calyx EOs, which presented MICs of 0.063% and 0.03%, respectively. In the study with the disk diffusion method, a halo of inhibition higher than that of the control groups was obtained. The effectiveness of the oils against the treated microorganisms, can be correlated with the chemical composition. The study with this plant is unprecedented and the results obtained are promising in the search for new antibacterial products.
Keywords: public health; antibacterial agents, Staphylococcaceae, Enterobacteriaceae, Asteraceae, volatile oils
This article explores the characteristics and chemical composition of essential oils obtained from different parts of the C. phyllolepis plant, including the leaves (Pt 1), the calyx (Pt 2), and the petals (Pt 3). Variation in color shade and yield was observed among these plant parts, suggesting significant differences in the oil composition. The objective of this article was to analyze the chemical composition of essential oils extracted from different parts of the C. phyllolepis plant and evaluate their antibacterial properties. To achieve this, the study identified the main compounds present in the oils and investigated their effectiveness against two bacterial strains, S. aureus and E. coli. The results obtained reveal the significance of these essential oils as potential antimicrobial agents and indicate the promising application of these compounds in the pharmaceutical industry, especially considering the growing concern about bacterial resistance to conventional antibiotics.
When entering the problem of public health, the pharmaceutical context stands out as a complex issue to be addressed, since every day new diseases and organisms resistant to drugs already available appear, making the need for discovery of new drugs more evident.1 Studies with natural products have boosted the synthesis of numerous molecules with antimicrobial activity, bringing gains to the present time.2 Microbiology is described as the science that studies living beings of microscopic dimensions. It appeared parallel and complementary to the invention of the microscope, a fact that marked great advances for biology, medicine, and many other areas of knowledge. Regarding microbiology as a science, one of the great advances was the discovery that microorganisms cause diseases in humans. This discovery promoted great changes in people's quality of life.3
The evolutionary capacity is intrinsic to living organisms, through which they are selected by virtue of adaptive characteristics to environmental conditions, and finally, through heredity, pass their mechanisms to the next generations.4 When it comes to microorganisms, this whole process occurs in an extremely reduced time scale, which associated to the intensive and indiscriminate use of compounds such as antibiotics, has promoted the emergence of resistance of organisms to the action of antibiotics already known, and consequently drives the studies in search of new compounds.5 And, although new antibiotics are being forged, they are not responding at the same speed as the appearance of strains resistant to their action, leading to an increase in the immunocompromised population.5 Some microorganisms stand out among the most problematic pathogens to human beings, for becoming resistant as Staphylococcus aureus and Escherichia coli .2
S. aureus belongs to the family Staphylococcaceae and is a Gram-positive bacterium native to the commensal flora of humans, often inhabiting the nostril and perineum.6 Morphologically it is similar to a bunch of grapes, fact from which derives the name. They are immobile, facultative anaerobes, grow at temperatures between 18ºC and 40ºC and are tolerable to high concentrations of salt. Among their characteristics, they are distinguished by causing plasma coagulation, due to the presence of the coagulase protein, is highly dangerous due to the combination of antibiotic resistance and their virulence factors.7
E. coli belongs to the family Enterobacteriaceae and is found in the environment as well as in the commensal flora of various animals, including humans. They are of the Gram-negative type, can be immobile or mobile, facultative anaerobes, glucose fermenters and without nutritional requirements, being known as coliforms. The strains can be divided into three groups: commensal, intestinal pathogenic and extraintestinal. It is a species with enormous clinical importance because besides presenting a wide variety of strains that develop in many parts of the human organism, it can cause several damages such as: urinary tract infections, meningitis of the newborn and hospital infections, which can vary according to the antigenic characteristics and specific virulence factors of each strain.8
A possible solution to the problem cited above, which has been explored, is the use of essential oils (EOs) in the production of pharmaceuticals. Their use is mainly due to their physical-chemical characteristics, which provide antiseptic and medicinal properties, used as antimicrobials, analgesics, sedatives and anti-inflammatories,5 and their effectiveness many times, is associated with the presence of alcohols, aldehydes and phenols or the synergistic action of different classes of compounds such as aldehydes, ketones, esters and hydrocarbons,5,9 encompassing among other activities cytotoxicity, carcinogenicity, mutagenicity and antimutagenic properties.9
The cytotoxic action is the main justification for the antimicrobial activity of the (OEs), however this does not resume the characteristic of the compound, but also to the state of the microorganism. The mechanisms can vary from the alteration of the permeability of the membrane, coagulation of the cytoplasm or even inhibition or inactivation of the metabolic processes of the fungus and bacteria, leading to cell lysis.3,5,10 These studies and visualizations were possible thanks to many techniques such as scanning electron microscopy (SEM), which allowed the observation of the pores and deformations in the bacterial cells evaluated with (OE).10
The EOs are the target of interest of the pharmaceutical industry for their chemical characteristics and biological potentialities and present from the function of providing natural compounds for semi-synthesis, to the direct use for the desired purposes, since an EO can be formed from dozens or even hundreds of compounds.11
Highlighted as producers of essential oils, the Asteraceae family comprises about 1,600 genera and 23,000 species worldwide, occurring mainly in subtropical and tropical regions,12 has numerous chemical compounds have already been isolated besides being used in the preparation of drugs.13 Researchers have reported antibacterial activity for (OE) of this family14 , as the antimicrobial activity for some species, such as Baccharis dracunculifolia D.C. and Baccharis uncinella D.C. on S. aureus and E. coli.14 For the genus Calea the literature presents several pharmacological applications, among which antimicrobial activity.15,16
Given the importance of discovering new sources of bioactive principles and the prominence of the genus Calea in the field of microbiology, this article sought to identify the chemical composition of the essential oils of a plant species empirically used as a remedy in Brazil, Calea phyllolepis Baker (Asteraceae), and to evaluate the sensitivity of the oils against resistant strains of pathogenic bacteria: Staphylococcus aureus (ATCC 29213) and Escherichia coli (ATCC 25922) by two methods, disk diffusion and microdilution. This plant is popularly called Amarelinha (Figure 1).17
Collecting the plant
C. phyllolepis was collected in Sidrolândia-MS. The material used for extraction was: leaves and flowers (calyx and petals). The exsiccate with testimonial plant material was deposited in the herbarium of UFGD (Federal University of Grande Dourados). The following are the collection data as geographical coordinates and deposit number (Table 1).
|
Date of collection |
Botanical Family |
Name of the plant |
Coordinates |
Herbarium Number |
|
10-04-18 |
Asteraceae |
Calea phyllolepis Baker |
21O 05 18,2" S |
6216 |
|
54O 32' 29.0" W |
||||
Table 1 Data regarding the plants under study
Oil extraction
Extractions were performed with Clevenger apparatus, with samples of 300 grams, for approximately four hours. The (OEs) obtained were stored in flasks, sealed for later use and kept under refrigeration in refrigerator at -4o C, with adapted methodology.18 Analytical balance (Químis model Q500L210C) was used for the weighings. The yields in percentage of the (OEs) were determined by the relation of the mass in grams of the fresh material and the mass of the oil, observing the volume obtained during the extraction process. To measure the mass a scale with precision of 10-4 g was used. The density of each oil sample was calculated using a 1mL pipette, measuring 0.1 mL of the sample and weighed on an analytical balance (Químis model Q500L210C). This test was performed in triplicate. The values were converted to g/mL and the final average was calculated.
Chemical analysis: Identification and quantification of compounds
For the structural elucidation of the compounds of the (OEs) chromatographic techniques were used: Gas Chromatography Coupled with Mass Spectrometer (GC-MS) and Gas Chromatography Equipped with Flame Ionization Detector (GC-DIC), having the same chromatographic conditions:
-GC-MS: performed with Thermo-Finnigan Focus DSQ II apparatus, with a quadrupole mass analyzer, electron impact ionization (70eV) and automatic sampler model Triplus. Essential oil and volatile separation was performed using a DB-5 capillary column (30m×0.25 mm I.D.×0.25𝜇m film thickness) with 5% phenylmethylpolysiloxane. Analytical grade 5.0 helium was used as carrier gas at a flow rate of 1.2 mL min-1 . The injector was operated in split/splitless mode for 5 min without flow splitting and the injection volume was 2.0 µL of the oil diluted in ethyl acetate. The GC temperature program used was 40∘ C (1 min) and 4∘ C min-1 up to 280∘ C. The injector, ionization source and transfer line temperatures were set at 230∘ C, 250∘ C and 280∘ C, respectively. In the TIC (total ion chromatogram) mode of operation the mass was varied from 50 to 500 amu (atomic mass units). Data reading was performed by Xcalibur 1.4 SR1 Software. Data analysis was performed by the NIST MS Search 2.0 library. -CG-DIC: was performed with equipment Model 6890N-Hewelett Packard-Agilent Technologies, Inc, operating with the software N2000, with N2 and H2 as carrier gas. Identification of the components was done by comparison of the mass spectra with the mass spectra available in the equipment database, with the literature and by Kovat's index, for which a mixture of standards of a homologous series of hydrocarbons C7-C30 (Sigma-Aldrich) were used.19,20
Bioassays
The bacteria evaluated in this article were Staphylococcus aureus (ATCC 29213) and Escherichia coli (ATCC 25922), using the microdilution and macrodilution techniques.
Minimum inhibitory concentration (MIC)
The dilution method was used to determine the minimum concentration for growth inhibition of microorganisms (MIC).21 Dilutions: with oil density of 0.9 g/mL, the following were added in sterile glass tubes: 0.8 mL of oil, 0.05 mL of Tween 80 and 4.2 mL of sterile distilled water at a concentration of 144 mg/mL (16%). In each well of the microdilution plates, 100 µL of Müller-Hinton broth was inserted, then 100 µL of the oil emulsions were inserted to obtain the initial concentration of 8% (72 mg/mL) in the first line of the microdilution plate. For each plate, 10 µL of the bacterial suspension of the strains shown in Table 1 was inserted. Subsequent concentrations of the oils were obtained after serial dilution in the plate itself, from the initial concentration of 8% (line A) to 0.004% (line L), by transferring 100 µL of the content of the subsequent well. For wells of line L, 100 µL of the content were dispensed to equalize the total volume of the wells. The analyses were performed in duplicate.
The toxicity control of Tween 80 at the concentration used for the emulsion was performed to verify that it had no inhibitory activity for the bacteria. The oil was also controlled to verify its sterility (oil plus culture medium). The positive control was a bacterial suspension in saline solution with turbidity corresponding to 0.5 of the McFarland Scale plus Polymyxin B (commercial antibiotic with inhibitory action for the microorganisms studied) and as a negative control, bacterial suspension plus culture medium. In the 96-well plates, the lines A to L were used, to obtain a greater series of dilutions for each sample tested. In each plate, the two bacteria were evaluated concomitantly, in column A (Staphylococcus aureus) and in column B (Escherichia coli), alongside the 4 control groups. The plates were incubated at 36ºC for 24h. For revelation of the results, 0.01% sodium resazurin was used. Next, colorimetric reading was performed, where blue staining demonstrates bacterial inactivity and red, bacterial activity, and the methodology was adapted according to Bonan PRF.22
Disk diffusion method
We used the agar disk diffusion method,23 accepted by the Food and Drug Administration (FDA) and established as a standard by the National Committee for Clinical Laboratory (NCCLS), to screen the antimicrobial activity of essential oils. The bacterial cultures were prepared by inoculating into Müller Hinton Agar (MHA) plates from the lyophilized strains using a sterile swab and an aliquot was removed and inserted into 4 ml of saline solution. It was then vortexed for ten seconds and the excess was removed from the edge of the tube with the swab and inoculated onto the plate, incubating the plates at 36ºC for 24 h. After 24 h, 4 to 5 colonies were removed and inserted in saline solution, and the turbidity of each culture was adjusted to an optical density similar to McFarland's 0.5.
Afterwards, total inoculation of the plate was performed taking care that all spaces were filled. For the disk diffusion assay, a sterile filter paper disk (6 mm diameter) impregnated with 5µL of (OE) was used, which was placed on the inoculated agar plate with slight pressure. Discs of chloramphenicol (75 g/disc) was used as the positive control, and sterile distilled water (5µL) was used as the negative control. All inoculated plates were incubated at 36°C for 24 h. After the incubation period, the halos formed were read using a digital pachymeter, comprising the diameter of the halo, considering the disk. The experiment was conducted in duplicate.
Characteristics and chemical composition of the oils
In this research, thirteen compounds were identified in the essential oils of C. phyllolepis, with high concentrations of Sabinene, (-)-α-Pinene, and ρ-Cymene. The oils demonstrated effective antimicrobial action against S. aureus and E. coli, with minimum inhibitory concentrations ranging from 0.063% to 4.0% and 0.03% to 8.0%, respectively. The oils from petals and calyxes stood out as the most effective. The results indicate the potential of C. phyllolepis oils for the development of antibacterial drugs, but further studies are required on resistant strains and in humans. As a result of the oil study among the parts of C. phyllolepis (leaves (Pt 1), calyx (Pt 2), petals (Pt 3), it was noticed variation in color shade and yield among the parts (Figure 2 & Table 2).
Figure 2 (OE) of C. phyllolepis in the extraction apparatus (Clevenger): (a) Part 1, (b) Part 3, and (c) Part 2.
|
Parts of plants |
Yield (%) |
Coloration |
|
Pt 1. |
0,32 |
Light Green |
|
Pt 2. |
0,26 |
Dark yellow |
|
Pt 3. |
0,06 |
Light yellow |
Table 2 Extraction data of C. phyllolepis
Thirteen compounds were identified in the EO of C. phyllolepis, being the same for each part of the plant. In the composition are present five monoterpenes and only one oxygenated, and eight sesquiterpenes, one of them is oxygenated. The quantitative behavior of these compounds varied in relation to the parts evaluated, being identified 80.02% for Pt 1, 91.32% for Pt 2 and 90.23% for Pt 3. The majority compounds of each part of the evaluated plant varied quantitatively. There is difference in the concentration of compounds and the chromatograms (GC-DIC) show the compounds eluted from each sample, with emphasis on major compounds (Figures 3–5& Tables 3,4).
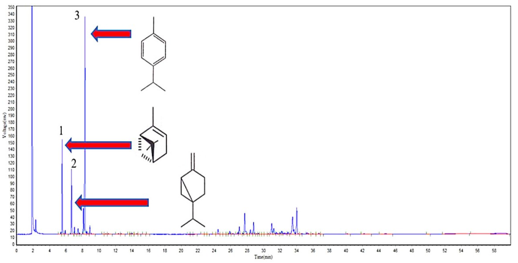
Figure 3 Cromatographic profile of (EO) from C. phyllolepis leaves, highlighting the major compounds: 1-(-)-α-Pinene (10.7%), 2-Sabinene (8.4%) and 3-ρ-Cymene (33.1%).
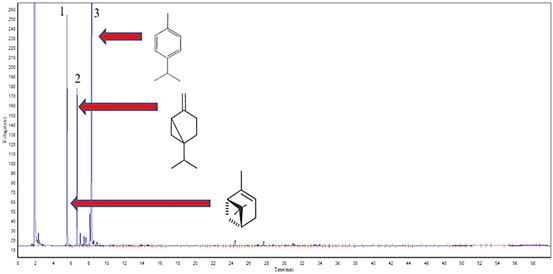
Figure 4 Cromatographic profile of (EO) from C. phyllolepis petals, highlighting the major compounds: 1-(-)-α-Pinene (21.3%), 2-Sabinene (16.7%) and 3-ρ-Cimene (48.8%).
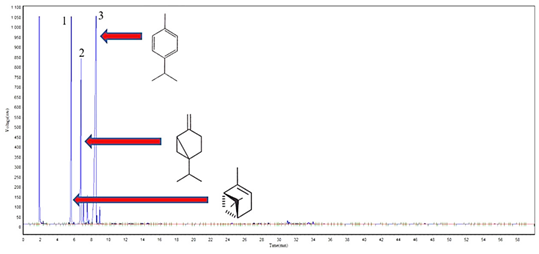
Figure 5 Cromatographic profile of the (EO) of the calyx of C. phyllolepis, highlighting the major compounds: 1-(-)-α-Pinene (20.2%), 2-Sabinene (15.8%) and 3-ρ-Cimene (49.6%).
|
* |
|
T. R. (min) |
Compound name |
F. M. |
Molar Mass |
IKC |
IKT |
|
1 |
|
5,523 |
(-)-α-Pinene |
C10 H16 |
136 |
913 |
939 |
|
2 |
|
6,673 |
Sabineno |
C10 H16 |
136 |
960 |
975 |
|
3 |
|
8,298 |
ρ-Cimene |
C10 H14 |
134 |
1043 |
1024 |
|
4 |
|
8,898 |
Limonene |
C10 H16 |
136 |
1046 |
1029 |
|
5 |
|
24,457 |
Caryophyllene |
C15 H24 |
204 |
1369 |
1418 |
|
6 |
|
25,890 |
Iso-verbanol acetate |
C12 H20 O2 |
196 |
1308 |
1309 |
|
7 |
|
27,048 |
(-)-α-Cubebene |
C15 H24 |
204 |
1341 |
1348 |
|
8 |
|
27,698 |
(-)-δ-Elemene |
C15 H24 |
204 |
1359 |
1388 |
|
9 |
|
28,782 |
(-)-β-Cubebene |
C15 H24 |
204 |
1389 |
1388 |
|
10 |
|
30,990 |
Spatulenol |
C15 H24 O |
220 |
1592 |
1549 |
|
11 |
|
31,223 |
(-)-α-Cubebene |
C15 H24 |
204 |
1389 |
1388 |
|
12 |
|
33,515 |
(-)-β-Cubebene |
C15 H24 |
204 |
1327 |
1388 |
|
13 |
|
34,023 |
(-)-α-Cadinene |
C15 H24 |
204 |
1543 |
1537 |
Table 3 Compounds identified for the (EO) of C. phyllolepis by GC-MS
Note: *-Number of identified compounds; MF, molecular formula; Tr, retention time; IKT, tabulated Kovatz retention index; IKC, calculated Kovatz retention index
|
Compound name |
Pt 1 (%) |
Pt 2 (%) |
Pt 3 (%) |
|
(-)-α-Pinene |
10,7 |
21,3 |
20,3 |
|
Sabineno |
8,4 |
16,7 |
15,8 |
|
ρ-Cimene |
33,1 |
48,8 |
49,6 |
|
Limonene |
1,7 |
1,4 |
0,7 |
|
Caryophyllene |
1,0 |
0,4 |
0,9 |
|
Iso-verbanol acetate |
0,7 |
0,1 |
0,2 |
|
(-)-α-Cubebene |
1,2 |
0,2 |
0,03 |
|
(-)-δ-Elemene |
5,0 |
0,8 |
0,9 |
|
(-)-β-Cubebene |
2,5 |
0,2 |
0,2 |
|
Spatulenol |
2,5 |
0,5 |
0,5 |
|
(-)-α-Cubebene |
1,7 |
0,3 |
0,4 |
|
(-)-β-Cubebene |
6,1 |
0,4 |
0,5 |
|
(-)-α-Cadinene |
5,5 |
0,3 |
0,3 |
|
Total income % Total income % Total |
80,0 |
91,3 |
90,3 |
|
income |
|
|
|
Table 4 Description of % variation of (EO) compounds as a function of different parts of C. phyllolepis
Biochemical and physiological alterations may occur that modify the production of biologically active substances in plants, affecting the quality, quantity and content of secondary metabolites, by necessity, and depending on external biotic factors such as injury caused by insects or fungi the time of collection, weather conditions, soil and other factors arising from the environment.24 Therefore, this information can be correlated with the variation in the composition of C. phyllolepis oils, which showed significant decreases and increases in the concentration of compounds between the evaluated parts. The compounds Sabinene and ρ-Cimene have high concentrations in C. phyllolepis. The literature lacks pharmaceutical studies on them, although the latter is already used in several areas, acting as an industrial solvent for paints and varnishes and production of synthetic resins. It is used in perfumery and as a thermal fluid.25
Minimum inhibitory concentration (MIC)
An effective action of the oils was observed against the two bacteria evaluated in the microdilution test, with the minimum concentrations to effectively inhibit the development of bacteria ranging from 0.3% to 4.0% against S. aureus and 0.3% to 8.0% for E. coli, observed in the colorimetric test, with blue coloration indicating absence of microbial growth, red indicating viable cells in growth, more intense blue indicates strong inhibition of bacterial growth.3
The most effective action against the bacteria studied were found for samples 2 and 3, referring to petals and calyx, which had an MIC of 0.063% and 0.03%, respectively. The leaf oil also showed promising results, but demonstrated selectivity, with better sensitivity against S. aureus, with MIC of 0.125% and MIC
These results highlight the plant species because its samples showed sensitivity in controlling bacteria even at the lowest concentrations tested. The oil from the leaves showed selectivity, with greater sensitivity to gram-positive bacteria, while the oil from the calyx and petals had effective action, even at the lowest concentrations, against the two strains evaluated (Figures 6–8). The Minimum Inhibitory Concentration (MIC) of the EOs tested against S. aureus and E. coli bacteria varied between 8.0% and 0.004% (Table 5).
|
Minimum inhibitory concentration (MIC) %. |
||
|
Sample-essential oils |
Staphylococcus aureus |
Escherichia coli |
|
1. C. phyllolepis-Leaf |
0,125 |
1,0 |
|
2. C. phyllolepis-Petals |
0,03 |
0,063 |
|
3. C. phyllolepis-Calyx |
0,03 |
0,03 |
|
4. Control: Polymyxin B |
0,004 |
0,004 |
Table 5 Minimum Inhibitory Concentrations for the two strains of bacteria analyzed
The disc diffusion method
The results obtained for the disk diffusion method corroborate those found in the microdilution, again highlighting the action of the calyx oil against the two bacteria, and for E. coli the zone of inhibition was 42.71 mm, a result superior to the control group (chloramphenicol disks with 34.75 mm of inhibition) and also showed a zone of inhibition very close to the value of the control group against S. aureus, with 49.43 mm of inhibition, against a value of 49.66 mm for the control group (Figures 9–11 & Table 6).
|
Sample-essential oils |
E. coli (mm) |
Control (mm) |
S. aureus (mm) |
Control (mm) |
|
1. C. phyllolepis-Leaf |
7,92 |
34,75 |
15,8 |
49,66 |
|
2. C. phyllolepis-Petals |
24,26 |
34,75 |
29,90 |
49,66 |
|
3. C. phyllolepis-Calyx |
42,71 |
34,75 |
49,43 |
49,66 |
Table 6 Growth measurements of the cultures submitted to the action of the (EOs) under study, in addition to the controls (chloramphenicol (75 g/disc)
The antibacterial results found for the leaves, petals and calyx of C. phyllolepis, are directly correlated with its chemical composition. The oils showed high concentrations of (-)-α-Pinene, ρ-Cymene and Sabinene, compared to other compounds of the plant, varying between the parts described, being in the calyx the highest concentration of these three constituents, with (21.26%), (48.8%) and (16.7%) respectively, which may justify its outstanding antibacterial action.
The most intense compounds found in the EOs of C. phyllolepis already have antibacterial action described in the literature for other plant species , describe the results against strains of S.aureus, and E.coli the presence of (-)-α-Pinene and correlates the action of the oil to the majority compound, as well as,26 and attribute the antibacterial action to (-)-α-Pinene as well as to its isomer the β-Pinene.3 Regarding the compound ρ-Cymene, it was involved in a study that shows that alone the compound has no action against bacteria, but attributed an important role to it, they described its action as a facilitator, showing that, acting in combination with other compounds that have bacterial action, the ρ-Cymene helps in the transport of these metabolites through the cytoplasmic membrane into the bacterial cell.27 These data may explain why the oil of C. phyllolepis showed sensitivity against both bacteria evaluated; it can be observed that the higher the concentration of the major compounds, the higher was its antibacterial activity. The compound Sabinene was described with important action against bacteria, both Gram-negative and Gram-positive28, corroborating the data obtained in this study, where the oil exhibited high antibacterial activity and broad spectrum of action, especially for the calyx that showed a concentration of 16.7% of Sabinene compound in relation to its total mass, presented above.
The antibacterial results are due to the synergistic action of the compounds present in EOs, and not to the individual action of the major components.29 Moreover, the antibacterial action is associated with the presence of oxygenated compounds with reduced molecular volume, for being able to establish hydrogen bridges and for its water solubility,30 and in the oil of C. phyllolepis two oxygenated compounds were registered (compounds 6 and 10).
It is believed that the results of this research contributed to the framework of knowledge of the studied species. The results of the oils evaluated in the control of S. aureus and E. coli may serve as a basis to guide important studies with prospection for the development of new methods of control of bacteria pathogenic to humans. The plant species C. phyllolepis stands out for its annual life cycle, being easy and fast to cultivate, for providing molecules from natural resources, which facilitates its excretion compared to synthetic compounds, reducing side effects, and improving the degree of therapeutic action.
C. phyllolepis has high concentration of compounds with bacterial action already proven in other studies as well as high concentrations and ρ-Cimene, compound that facilitates drug-bacterial cell interaction.
The chemical and biological study with this plant is unprecedented, presenting new data for the species and for the control of these bacteria, which are becoming resistant to drugs that have been used in their control.
The species C. phyllolepis holds great importance for the pharmaceutical industry due to the promising results of its essential oils against pathogenic bacteria such as S. aureus and E. coli. Compounds present in the oils, such as Sabinene and ρ-Cymene, have demonstrated effective antimicrobial activity at low concentrations. Its rapid cultivation and sensitivity to low concentrations make this plant a promising source of bioactive compounds for the development of new antibacterial drugs, particularly relevant amid the growing concern over bacterial resistance to antibiotics.
None.
There are no conflicts of interest presented or declared by the authors in this research.
None.

©2023 Mallmann, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.