Journal of
eISSN: 2572-8466


Research Article Volume 2 Issue 3
1Department of Biochemistry, Federal University Wukari, Nigeria
2Department of Biochemistry, University of Nigeria, Nigeria
3Department of Public Health, Federal Ministry of Health, Nigeria
Correspondence: Tatah Verwiyeh Silas, Federal University Wukari, Department of Biochemistry, Taraba State, Nigeria, Tel 2348065982609
Received: October 31, 2016 | Published: March 3, 2017
Citation: Tatah VS, Otitoju O, Ezeonu CS, et al. Characterization and adsorption isotherm studies of Cd (II) and Pb (II) ions bioremediation from aqueous solution using unmodified sorghum husk. J Appl Biotechnol Bioeng. 2017;2(3):113-120. DOI: 10.15406/jabb.2017.02.00034
Contamination of environment by heavy metals has become a major concern worldwide. The role of conventional methods in remediating heavy metals has become ineffective and costly. Therefore, it becomes imperative to explore bioremediation using a cost effective, efficient and environmentally friendly alternative method of removing heavy metals. In this study, the adsorption behavior of Sorghum husk, a low-cost adsorbents, with respect to Pb+2 and Cd2+ ions, has been studied in order to consider its application to the treatment of wastewater. The physicochemical properties of the unmodified sorghum husk were predetermined. The batch experimental method was employed: parameters such as pH, contact time, particle size and initial metal concentration were studied. The influence of the pH of the metal ion solutions on the uptake levels of the metal ions by the adsorbent used was carried out between pH 3 and 8. The optimum pH for Pb+2 and Cd+2 removals by sorghum husk were 6 and 5 respectively. An equilibrium time of 80min was required for the adsorption of Pb+2 and 60min for Cd+2 ions onto sorghum husk. Percentage sorption of Pb+2 increased from 21 to 80% and that of Cd+2 from 15 to 55% as adsorbent dose was increased from 0.1 to 1g while adsorption capacity decreased from 14 to 5.3mg/g for Pb+2 and 32.5 to 9.3mg/g for Cd+2 with increase in dose. Percentage removal of Pb+2 increased from 24.8 to 93.7% while that of Cd+2 from 22.4 to 91.6% with decrease in adsorbent particle size from 1000μm to 100μm. The uptake capacity of the adsorbent increased from 6.5 to 12.4mg/g for Pb+2 and 2.1 to 5.3mg/g for Cd+2 as the initial metal ion concentration was raised from 50 to 200mg/L. The experimental data was fitted into the Langmuir and Freundlich models; the data was best fitted with the Langmuir model. The results demonstrated that sorghum husk holds potential to remove cationic heavy metal species from industrial wastewater.
Keywords: bioremediation, adsorption, wastewater, heavy metals, isotherms
CA, conventional adsorbent; NA, natural biosorbent; MB, modified biosorbent; SH, sorghum husk; SEM, scanning electron microscopy
Pollution of the environment with toxic substances in wastewater effluents is a major concern for public health and environmental quality, with heavy metals being one of the most dangerous pollutants. These have rendered many waters unwholesome and hazardous to man and other living resources.1 The release of these heavy metals posses a significant threat to the environment and public health because of their toxicity, bioaccumulation in the food chain and persistence in nature.2-3 It has been reported that the toxicity due to metallic discharges into the environment far exceeds the combined total toxicity of all radioactive and organic waste.4 Metallic effluents can lead to increased nutrient load in water bodies especially if they are essential metals.5 Also, these metals in effluent may increase fertility of the sediment and water column and consequently lead to eutrophication, which in open waters can progressively lead to oxygen deficiency, algal bloom and death of aquatic life.2,3
Consequently, the treatment of heavy metal contaminated wastewater remains a topic of global concern since wastewater collected from municipalities, communities and industries must ultimately be returned to receiving waters or to the land.6 Conventional treatment methods have been found to be very expensive, and difficult to maintain due to high capital and operational costs 7,8 and also results in the generation of chemical sludge/secondary waste that must be treated before disposal as it also poses hazards and pollution risks to the environment.9 These challenges associated with conventional methods, have triggered interest and research for more efficient and eco-friendly heavy metal treatment methods like adsorption. Adsorption process involves the use of low-cost, naturally-occurring and readily available organic or agricultural waste materials as adsorbents and has been reported in various studies to be effective in the treatment of metal-contaminated wastewater.10 The distinctive advantages of adsorption include: low cost, high efficiency, reduced production of chemical or biological sludge, possibility of regeneration of adsorbents and metal recovery.11 The increased biomass level of sorghum husk (SH) in the environment through dumping as refuge due to high consumption rates of these agricultural products has become an environmental concerned due to their land space occupation and subsequent pollution problems. Hence the recycling of this agro-waste for potential use in remediation of Pb(II) and Cd(II) ion contaminated wastewater through adsorption process as an alternative treatment method has been investigated in this study.12
Preparation of adsorbent
Sorghum husk (SH) was obtained from a local farm in Wukari local government area of Taraba state, Nigeria and identified at the Department of Botany, University of Nigeria, Nsukka. The SH was then washed with tap water and then rinsed with distilled water to remove dust and impurities deposited on the surface. They were further air-dried and then oven dried at temperature of 80°C to a constant mass. The dried biomass was then pulverized and sieved using an electromagnetic sieve to obtain particle sizes less than 250μm. The dried biomass was then preserved in air-tight polyethene paper to protect it from moisture and make ready for analysis.13 The adsorbent was used in their natural form without any form of modification.
Preparation of Stock Solutions
Spectroscopic grade chemicals were used in preparing all the solutions that were used in the study. Synthetic stock solution of Cd (II) and Pb(II) effluent (1000 mg/l) were prepared by dissolving separate 1.62 g of CdCl2 and 1.59g of Pb(NO3)2 in 1000 ml of deionized water. All other concentrations of the metal ions were prepared by serial dilution of the stock solutions. Hydrochloric acid 0.1 M and 0.1 M NaOH was used for adjustment of the aqueous solution pH.
Scanning Electron Microscopy
Scanning electron microscopy (SEM; Hitachi S4800) was carried out to assess the morphology of the adsorbent, which was done before and after adsorption.
Determination the moisture content
This was done by weighing 5g of SH into a crucible, then placed in an oven and heated for 5hours at constant temperature of 105°C. The sample was then removed and put rapidly into a desiccator in order to prevent moisture uptake from atmosphere. The sample was re-weighed. This procedure was repeated several times until a constant weight was obtained. The difference in the mass constitutes the amount of moisture content of the adsorbent.14
(1)
W1 = Weight of crucible
W2 = Initial weight of crucible with sample
W3 = Final weight of crucible with sample
The determination of loss of mass
The determination of loss of mass on ignition was done by weighing 10g of the adsorbent (SH) and put in the furnace at constant temperature of 600°C for 2hours. After roasting, the sample was removed and put in a desiccator for cooling. The residual product was then weighed and the difference in mass represented the mass of organic material present in the sample. This operation was repeated four times.
The Determination of bulk density
The bulk density of each of the samples of SH was determined using Archimedes‘s principle by weighing a 10cm3 measuring cylinder before and after filling with the samples. The measuring cylinder was then dried and the sample was packed inside the measuring cylinder, leveled and weighed. The weight of the sample packed in the measuring cylinder was determined from the difference in weight of the filled and empty measuring cylinder. The volume of water in the container was determined by taking the difference in weight of the empty and water filled measuring cylinder. The bulk density was determined using the equation below.14
(2)
Batch Equilibrium adsorption Studies
Batch experiments were carried out in 100ml plastic bottles according to the methods described by Nharingo and Hungo 15 and Dawodu and Akpomie,16 to investigate how critical experimental parameters affects the adsorption process. The critical parameters investigated in this study include effects of pH, contact time, dosage, particle size and initial metal ion concentrations. Solution temperature, volume of synthetic effluent and rate of agitation were maintained constant throughout the study at 25±2°C, 50 ml and 180 rpm for 120min respectively.
Effect of adsorbent Dosage on adsorption
To each separate 50 ml portion of 200 mg/l of Cd (II) and Pb (II) ions solutions in a 100ml plastic bottle, varying amounts of biomass ranging from 0.1 to 1 g were separately added. The mixtures were agitated over a shaker at 180 rpm for 120 mins at a temperature of 25±2°C, pH of 6.0. The mixtures were finally filtered and analyzed for residual metal ions by an atomic absorption spectrophotometer (Buck Scientific, model 210VGP).
Effect of pH on adsorption
The effect of pH on the percentage removal Cd(II) and Pb (II) ions by SH was investigated using Cd(II) and Pb (II) ions aqueous solutions of initial concentration 200 mg/l. Separate volumes of 50 ml of 200 mg/l of Cd(II) and Pb (II) ions were transferred into separate 100 ml plastic bottles. The pH was adjusted between 3.0-8.0, using 0.1 M HCI and 0.1M NaOH solutions. 1g SH, was added to each plastic bottles containing metal solutions of varying pH and the mixtures were placed on an electronic shaker operated at 180 rpm for 120 mins at temperature of 25±2°C. The samples were finally filtered and analyzed for residual metal ions using an atomic absorption Spectrophotometer (Buck Scientific, model 210VGP).
Effect of initial metal concentrations on adsorption
The effect of initial concentrations of metal ion on adsorption was examined under optimum conditions by mixing 1g SH powder with 50 ml of Cd(II) and Pb (II) ion solutions of different initial concentrations ranging from 50 to 200 mg/l at pH of 6.0. The experiments were conducted at a temperature of 25 ±1°C, shaking rate of 180 rpm for 120 mins. The samples were finally filtered and analysis for residual metal ions by an atomic absorption spectrophotometer (Buck Scientific, model 210VGP).
Effect of contact time on adsorption
Batch adsorption studies were conducted at different contact times from 5 to 120 minutes, by contacting 50 ml of 200 mg/l of Cd(II) and Pb (II) ions with 1.0g adsorbent dose at pH of 6.0, flask shaking rate of 180 rpm and room temperature of 25±2°C. Replicate analysis of the residual metal ions was done at each predetermined time by an atomic absorption spectrophotometer (Buck Scientific, model 210VGP). The equilibrium time was found to be 60 minutes for Cd(II) and 80 for Pb(II). Hence, 120 mins was used for subsequent experiments to ensure that equilibrium was achieved.
Effect of adsorbent particle sizes on adsorption
To each separate 50 ml portion of 200 mg/l of Cd(II) and Pb (II) ions solution in 100 ml plastic bottle, biomass particles of different sizes ranging from 1000 to <250μm were separately added. The mixtures were agitated over a shaker at 180 rpm for 120 mins at a temperature of 25±2°C, pH of 6.0. The mixtures were finally filtered and analyzed for residual metal ions an atomic absorption spectrophotometer (Buck Scientific, model 210VGP). In each experiment, one parameter was varied while the others were kept constant. The solution was filtered at the end of a given contact time, and the concentration of metal ions remaining in the filtrate was determined by an atomic absorption spectrophotometer (AAS) Buck Scientific, model 210VGP. Each experiment was performed twice; and the mean value was computed to minimize error. The amount of metal ions sorbed per unit mass of the adsorbent was calculated using equations 1 and 2:17
(3)
Where,
Q= the amount of solute adsorbed from the solution
V = Volume of the adsorbate
Ci = the concentration before adsorption
Ce = the concentration at equilibrium and
W= the weight in gram of the adsorbent.
The removal efficiency was determined by computing the percentage sorption using the formulae in Eq. (2).18
(4)
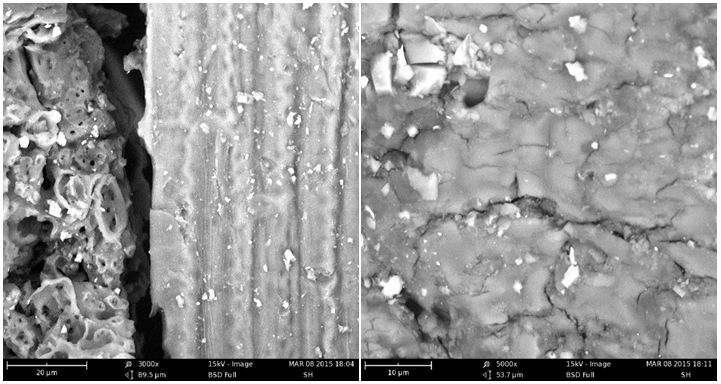
Characterization of the adsorbents before and after adsorption process was carried out to determine their suitability. The results of the FTIR spectra showed the participation of carbonyl, carboxylic and hydroxyl groups of sorghum husk as major binding sites for the binding of positively charged ions during adsorption as shown in Figure 1 & 2. A broad peak at 3431.48 cm-1 is the indication of -OH and -NH groups. The stretching of the -OH groups bound to methyl radicals is attributed to a signal at 2928.04 cm-1. The peaks at 1644.37 and 1529.60 cm-1 are characteristic of carbonyl group stretching from aldehydes and ketones. The presence of -OH group, along with carbonyl group, is attributed to the presence of carboxylic acid groups in the adsorbent. The peak observed at 1050.35 cm-1 is due to C-O bonds. After adsorption there was a shift and broadening of adsorption peaks. The shift of the -OH peak from 3431.48 to 3436.34 cm-1 indicates the involvement of the hydroxyl groups in the adsorption of Cd(II) and Pb(II). While the shifting of the carbonyl group peak from 1644.37 to 1649.19cm-1 also show that the carbonyl groups participated in the adsorption of Cd(II) and Pb(II). Similar spectra have been reported for adsorption studies of metal ions using groundnut hull,13 cashew nut shells 19 and palm nut shells.20 SEM analysis of unmodified sorghum husk before and after adsorption is shown in Figure 3A & 3B, which revealed the porous nature of the adsorbents and an increase in surface porosity, such that physical adsorption played a major role in the overall sorption process.21
The physico-chemical parameters of SH viz; pH, % Moisture Content, % Loss of mass on ignition, Bulk density (g/cm3), Particle sizes and pore size (μm). The values reported are in the range with those reported in the literature.14,22 The adsorption of heavy metals from wastewater is influenced by several physical and chemical factors, such as pH, temperature, initial heavy metal concentration, adsorbent dose, adsorbent particle size, ionic strength, co-ions, etc. These factors determine the overall adsorption process by affecting the metal uptake rate, selectivity and amount of heavy metals removed.8 Adsorbent dosage has proved to be a useful parameter for determining the capacity of a adsorbent for a given initial metal ion concentration. The percentage removal of Cd(II) and Pb(II) ions in this study, increased with increasing adsorbent dosage (Figure 4).23-25 Increasing the dose from 0.1 to 1.0 g led to an increase in percentage removal of Cd(II) from 34.3 to 65.0% on BGH and 21.6 to 61.7% while percentage removal of Pb(II) increased from 21.0 to 80.0% on SH . This is due mainly to an increase in the number of available exchangeable binding sites for metal ion sorption.26,27 The equilibrium sorption capacity per unit mass of the adsorbents decreased considerably with increase in sorbent dose for both metal ions (Figure 5).28 Increase in biosorbent dosage led to a decrease in the equilibrium sorption capacity of Cd(II) from 32.5 to 9.3 mg/g on while that of Pb(II) decreased from14.0 to 5.3mg/g on SH. This may be due to a decrease in the total sorption surface area available to the metal ions, possibly caused by the aggregation/agglomeration of sorption sites, as a result the sorption capacity of the adsorbent is not fully utilised.29,30 Conversely, a higher adsorbent dose may provide more active sorption sites, so that adsorption sites remain unsaturated during sorption, leading to their full utilization.31,32
The percentage removal and equilibrium sorption capacity of metal ions is strongly affected by pH.33 At the same time, the state of chemically binding sites is changed by the solution pH .16 Sorption of both metal ions increased with increasing pH, significant percentage removal (65.1 to 92.3 %) occurred at pH 6-8 (Figure 6). An optimum pH of 6.0 was achieved for both Cd (II) and Pb(II) with percentage removal of 83.0% and 85.7% respectively on SH. At low pH, higher concentration and mobility of H+ ions favours H+ sorption compared to metal ions, this creates a competition between the protons and metal ions for the binding sites of the adsorbent. According to Onundi et al. 20 metal ions are more soluble in solution at lower pH values and this reduces their sorption. The low sorption at low pH in this study (Figure 6) was therefore due to saturation of binding sites of sorghum husk with hydrogen ions. However, an initial metal sorption observed with increase in pH was due to a decrease in competition between hydrogen ions and metal ions for the adsorbent surface binding sites and also due to decrease in positive surface charge, which resulted in less electrostatic repulsion between the surface and metal ions before ion exchange which is the major mechanism of metal uptake.20 As the pH of the solution increases, more negatively charged surface becomes available thus facilitating greater metal adsorption. Similar tendencies were found in adsorption processes using diverse agricultural waste biomass (AWBs). Giri et al. 34 reported a similar trend on studies of the effect of pH on the removal of Cr (VI) using Eichhornia crassipes root activated carbon. However, at higher pH metal ions tend to precipitate out of solution. Therefore the removal of metal ions at higher pH values is due to the formation of metal ion precipitates rather than sorption.35,36
The amount of metal ions sorbed is a function of the initial concentration of the metal ion, making it an important factor in effective adsorption. The sorption capacity of adsorbent (SH) for metal ions sorption in this study increased with increasing metal ion concentration. In Figure 7 the sorption capacity of Cd (II) and Pb(II) increased from 2.1 to 5.3 mg/g and 6.5 to 12.4 mg/g respectively as metal ion concentrations was increased from 52.5 to 201mg/L. This resulted to an increase metal ion concentration gradient which overcomes the resistance to mass transfer of metal ions between the aqueous phase and the adsorbent.37 A higher concentration in a solution implies a higher concentration of metal ion to be fixed on the surface of the adsorbent.31 Dawodu FA et al. 16 reported a similar observation on simultaneous adsorption of Ni(II) and Mn(II) ions from aqueous solution onto a Nigerian kaolinite clay. High metal concentration saturates the adsorbent sites more quickly thereby decreasing the overall percentage metal removal. A similar trend was observed by Kannan N et al. 38 The removal rate of metal ions also increases with an increase in contact time (Figure 8). The rate of adsorption is higher at the early stage, due to a large available surface area on the adsorbent and presence of abundant binding sites on the surface. The fast initial uptake is also due to the rapid accumulation of the heavy metal ions on the surface of the adsorbent. As these sites become exhausted or saturated with time the sorption rate also decreases. In this study Equilibrium sorption was achieved within 60 minutes for Cd(II) and 80 minutes for Pb(II) ions in the batch process, after which the rate of sorption became fairly stable. According to Dawodu FA et al..16, during initial stages, sorption is controlled mainly by diffusion from the bulk solution to the surface of the adsorbent, whereas subsequent sorption rate is controlled by the rate at which the adsorbate is transported from the exterior to the interior sites of the adsorbent particles, hence more time will then be consumed on diffusion of metal ions to binding sites which is probably an attachment-controlled process due to the presence of fewer binding sites.10 The faster removal rate of Cd(II) than Pb(II) may be due to the smaller ionic radius of Cd(II) (0.91°A) than Pb(II) (1.91°A), which makes for easier, more rapid diffusion to the surface of the adsorbent.16
The particle size of an adsorbent has a tremendous effect on the adsorption process.26 In this study decreasing the particle size from 1000 to <250 μm increased the percentage removal of Cd(II) 22.5 to 91.6% while percentage removal of Pb(II) increased from 24.8 to 93.7% on SH (Figure 6). The increase in the percentage removal of Cd(II) and Pb(II) ions with decreasing adsorbent particle size in this study is attributable to a decrease in the surface area of adsorbent available for metal ions binding (Figure 9). The breaking up of larger particles into smaller ones tends to open tiny cracks and channels on the particle surface of the adsorbent, resulting in greater accessibility and better diffusion of the metal ions16 Similar results have been reported previously.31,37,4
Equilibrium isotherm modeling
Equilibrium adsorption isotherms are used to relate the adsorbate concentration in solution and the amount on the adsorbent at equilibrium.39 These parameters often provide fundamental information on the sorption mechanism, surface properties and the affinity of adsorbents, which helps to determine the applicability of sorption as a unit operation. Therefore, it is important to establish the most suitable correlation of equilibrium curves in order to optimize the conditions for designing adsorption systems. The most frequently used isotherms, the Langmuir and Freundlich models, were therefore used to analyse the data (Table 2).
Properties |
Sorghum Husk |
pH |
6.0- 6.4 |
% Moisture Content |
11.5 |
% Loss of mass on ignition |
0.8 |
Bulk density (g/cm3) |
0.366 |
Particle size |
<250μm |
Pore size |
10 μm |
Table 1 Some physico-chemical parameters of the unmodified SH.
Isotherm model |
Sorghum husk (SH) |
|
Cd (II) |
Pb (II) |
|
Langmuir model |
||
qL (mg/g) |
6.993 |
58.82 |
KL (L/mg) |
0.041 |
0.003 |
R2 |
0.994 |
0.967 |
RL |
0.107 |
0.349 |
Freundlich model |
||
KF (L/g) |
0.114 |
0.567 |
1/n |
0.446 |
0.187 |
N |
2.242 |
5.347 |
R2 |
0.976 |
0.821 |
Table 2 Langmuir and Freundlich Isotherm constants for the adsorption of Cd(II) and Pb(II) ions unto Sorghum husk.
The Langmuir isotherm is used to describe monolayer sorption onto the surface of an adsorbent with a finite number of identical adsorption sites and no interaction between sites. The model is expressed as.40
(5)
Where,
qL(mg/g)= monolayer adsorption capacity of the adsorbent
KL(L/mg)= adsorption constant, which reflects the affinity between the adsorbent and adsorbate. qL and KL were determined from the slope and intercept of the plots of Ce/qe versus Ce.
It was observed in this study that the Langmuir adsorption isotherm provided a very good fit for the sorption of Cd(II) and Pb(II) ions from aqueous solutions on sorghum husk as indicated by the high correlation coefficients (R2) 0.994 and 0.967. The applicability of the Langmuir isotherm indicated good monolayer coverage of Cd(II) and Pb(II) ions on the surface of the adsorbent, which consequently suggests the formation of a mono layer on the adsorbent surface in the given concentration range.31 The fact that the Langmuir isotherm fitted the experimental data well may be attributed to the homogenous distribution of binding sites on the adsorbents.16 The calculated model parameters with their correlation coefficients are shown in Table 2. The adsorption capacity qL (mg/g) for cadmium (6.993mg/g) was lower than that of lead (58.82mg/g), suggesting that the amount of Pb(II) sorbed per unit mass of the adsorbent was higher than that of Cd(II). Qaiser et al. 13 reported the maximum sorption capacities for lead on groundnut hull (31.54 mg/g) and baggase fly ash (2.5 mg/g).41,42 Table 3 show the comparison of Sorghum husk with other agricultural and commercial adsorbents using their maximum adsorption capacity (qmax-mg/g) for the adsorption of Cd(II) and Pb(II). The affinity of the two metals for the adsorbent surface in terms of KL(l/mg) is higher for cadmium than lead. The values of KL(L/mg) were fairly low, which implies low surface energy in the process and consequently weak bonding between metal ions and adsorbent (indicating a physisorption mechanism) marking recovery of the metal ions through desorption easy. This is a major criterion in selecting a adsorbent.43 The Freundlich isotherm is based on the assumption that sorption takes place on a heterogeneous adsorbent surface, where the sorption energy distribution decreases exponentially and is expressed in equation 6:
(6)
The constant KFis an approximate indicator of adsorption capacity, while 1/n is a function of the strength of adsorption in the adsorption process.44,45 If n = 1 then the partition between the two phases are independent of the concentration. If value of 1/n is below one it indicates a normal adsorption. On the other hand, 1/n being above one indicates cooperative adsorption. However, KF and n are parameters characteristic of the sorbent-sorbate system, which must be determined by data fitting and whereas linear regression is generally used to determine the parameters of kinetic and isotherm models. A plot of log qe versus log Ce gives a straight line of slope 1/n and intercepts log KF .46 It was observed in this study that the Freundlich sorption isotherm provided a poor fit to the experimental data compared to the Langmuir model. This was indicated by the correlation coefficient values 0.976 and 0.821 on SH for Cd(II) and Pb(II) respectively as shown in Table 2. The values of n (5.347 and 2.242) for Cd(II) and Pb(II) on SH respectively, confirms that the adsorbent have a heterogeneous surface since the values satisfy the heterogeneity condition where n must be between one to 10 (1< n< 10).16 The low values of KF for both heavy metals imply that there was low uptake of the metal ions onto the adsorbent surface. Barka et al.31 also reported a low KF values for cadmium and lead uptake onto eco-friendly dried Cactus cladodes.
Metal Ion |
Adsorbent |
Type of Adsorbent |
Qmax (mg/g) |
Reference |
Cd (II) |
Commercial activated carbon |
C.A |
0.7 |
30 |
Granular activated carbon |
C.A |
1.39 |
36 |
|
Coffee grounds |
NA |
15.65 |
36 |
|
Rice husk |
MB (NaOH) |
20.24 |
37 |
|
Cashew nut shell |
NA |
22.11 |
38 |
|
Pineapple peel fibre |
MB (Succinic anhydride) |
34.18 |
39 |
|
Orange peel |
MB (Mercapto-acetic acid) |
136.05 |
40 |
|
Cashew nut shell |
MB (H2SO4) |
436.7 |
41 |
|
Sorghum husk |
NA |
6.7 |
Present Study |
|
Pb (II) |
Commercial activated carbon |
C.A |
5.9 |
42 |
Kaolinite |
CA |
7.75 |
43 |
|
Chitosan immobilized on bentonite |
CA |
15 |
44 |
|
Portulaca plant biomass |
NA |
17.5 |
42 |
|
Cocoa pod husk |
NA |
20.1 |
45 |
|
Pine cone powder |
MB (KOH) |
32.26 |
46 |
|
Cortex orange waste |
NA |
76.8 |
47 |
|
Rose petals waste |
NA |
119.92 |
48 |
|
Sorghum Husk |
NA |
58.82 |
Present Study |
Table 3 Comparison of Sorghum husk with other adsorbents using the maximum adsorption capacity (qmax-mg/g).
The adsorption of Cd and Pb by sorghum husk has been investigated in this study and has been found to be influence by pH, initial metal concentration, adsorbent particle size, adsorbent dosage and contact time. The FTIR spectra showed that certain functional groups are responsible for binding the metal ions from solution. SEM analysis also indicated the porous nature of the adsorbent. The experimental data was analyzed using the Langmuir and Freundlich models with the data fitting best with the Langmuir model. Further studies should be carried acid/basic modified sorghum husk because literature study on other adsorbent has shown modification drastically improve adsorptive capacity. It could be concluded from this study that unmodified sorghum husk is a potential adsorbent for possible remediation of wastewater contaminated with cadmium and lead ions.
None.
The author declares no conflict of interest.

©2017 Tatah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.