Journal of
eISSN: 2572-8466


Review Article Volume 10 Issue 1
California State University Channel Islands, USA
Correspondence: Bill Tawil, California State University, Channel Islands, USA
Received: January 19, 2023 | Published: January 30, 2023
Citation: Krol J, Tawil B. Cell-based meat: farming from a fermenter. J Appl Biotechnol Bioeng. 2023;10(1):17-22. DOI: 10.15406/jabb.2023.10.00322
Earth as we know it is not the planet that it was once on-track to become, prior to the intervention of the ancestors of humans. For millions of years humans and their predecessors have been using tools to shape their world in order to gain an evolutionary advantage over competing species.1 By shaping the world in such a way, the human population has managed to grow exponentially, and overcome pandemics and natural disasters consistently, swelling to a projected population of 9.7 billion by the year 2050.2 Along with a larger population comes greater carbon emissions, as well as a greater demand for land, food, and clean drinking water. Unfortunately, current methods of meat cultivation, an increasingly relied upon source of food globally,3 require massive amounts of both land and water. Currently, 77% of habitable land on the planet is used for maintaining livestock.2 Fortunately, an innovation in the fields of food sciences and biotechnology have presented the world with a possible resolution to this daunting future that faces the human population. As of November 16, 2022 the United States Food and Drug Administration (FDA) approved the use and sale of the first lab-grown, or cultured, chicken cells within food products for human consumption within the United States.4 Though this is the only currently approved product of its type, many companies are working towards similar goals, with this approval only being the first in a wave of similar products to come.5 This date is a monumental landmark in the advancement in the field of sustainable food products. Furthermore, the use of cultured meat products could offer solutions to massively reduce the carbon footprint and overall resource consumption of the meat industry as a whole.6 These promising advances could be pivotal to the continued growth and maintenance of the human population, but ultimately it is the commercial viability that will drive the widespread adoption of this novel production method. With dozens6 of companies pursuing similar advancements, and a forecasted compound annual growth rate of 15% from 2022-2030,8 this technology has the potential to disrupt the meat industry, and provide an ethical and sustainable source of meat worldwide.
Manufacturing process
Due to the infancy of the cultured meat industry, various techniques for producing meat within a laboratory environment, from start to finish, are being investigated by various companies. Generally speaking, the goal for the production process of cultured meat is to provide a minimal number of select animal cells to a cell culturing pipeline in which they can replicate to occupy high volume or high density.2 These cultures are then harvested and given shape using various scaffolding techniques.2,8 To achieve this, the production process is divided into four distinct steps which are subject to a high degree of variation as the technology evolves: animal tissue selection, cell-type and growth media selection and isolation, cultivation, and tissue engineering.2,9
Animal tissue selection is a relatively straightforward variable in the production process, as it is the variable that dictates the type of meat that will be produced. Traditionally produced meat products are made up of skeletal muscle cells, adipocytes which make up the fatty tissue, fibroblasts or chondrocytes that make up the connective tissue, and endothelial cells which make up the vascularization.10 Within animal tissue, these cells would differentiate themselves based on cellular signaling, and their structure would be dependent on their location within the organism as well as the organism’s genetic markers and physical demands.11 Therefore, the composition of the meat product that is harvested from the animal would be based on which animal type is selected, which part of the animal is harvested, and the animal’s bloodline and lifestyle. However, when designing a cultured meat pipeline for a specific type of meat, the only concern is the animal type selected.12 The type of animal selected will dictate the complexity involved in harvesting the desired cell type from a host organism, with all harvesting techniques demonstrating what is largely considered to be better ethical standards than traditional farming methods.6,9,13 Researchers are able to utilize different media types to then proliferate cells and force expression of different phenotypes, within the cell cultures with which they are working.10 In doing so, the desired cell type can be reliably produced.10 This is to say that the same sample of the desired animal cells would be capable of producing analogs for any cut of meat present within the specified animal.
Though the animal tissue selection is able to be non-specific based on the targeted end product, the selection of the cell type being used can have a significant impact on downstream variables.2 Methods of producing cultured meat have been based around three core cell types including myogenic satellite cells, embryonic stem cells, and induced pluripotent stem (iPS) cells.9 All of these techniques are, in theory, capable of producing cells with identical proteomes, though myogenic satellite cells are most commonly used for their elevated efficiency in forming varied complexes of muscle tissue.9 Selection of the cell type will also drive the media composition. In general, the growth media is used to encourage the proliferation of the selected stem cells and generate a high density of undefined stem cells that can later be induced to encourage differentiation in phenotype.2 Media composition details will likely need to be experimentally defined, as the current a standard additive for mammalian stem cell cultivation, fetal bovine serum (FBS), is highly variable, a source of possible contamination, difficult to source, and contradictory to the goal of animal welfare.2,12 The complex nature and cost of formulating and producing such a media is currently one of the aspects of cultured meat that is prohibitive to its mass production and adoption.2 Though the technology is vastly different than that used within the production of recombinant proteins within bacterial cells, the concept is the same; grow the cells until they are rapidly dividing towards a high density, then induce them in order to promote the production of a desired product.
Similar to selection of cell type, the selection of the culturing method will also greatly influence the efficiency by which cell mass is able to be propagated, and the downstream processing steps that will need to be taken to generate a final product.9,14 Researchers working to improve and scale the production efforts of cultured meat, however, have largely experienced complications in producing enough cell mass to confidently prepare for production-scale manufacturing.2,15 Efforts towards cultured meat are still exploring continuous stirred-tank, rocking platform, air lift, and vertical wheel bioreactors as possible solutions to these issues, though they each pose unique challenges.14 Both vertical wheel and rocking platform bioreactors are generally less economical, and though they offer great utility to the biopharmaceutical industry, the lower batch value of cultivated meat would not support the scaling of such options.14 Stirred tank bioreactors are generally a flexible option for biopharmaceutical production, however, they appear to have a maximum effective volume of 20,000 L, which may prove to be too small to support the needs of the food industry. Additionally, the heterogeneity of shear forces within a stirred tank environment16 could prove to be antithetical to the formation of the desired myotubes.12 Not bound by the same restrictions of heterogeneous shear forces of stirred tank reactors, air-lift bioreactors16 offer the potential of an undefined maximum scale, with the largest ever produced reaching 1,500,000 L (1,500 m3).14 A scale-down experiment that explored animal cell culturing for cultured meat production referenced and optimized this bioreactor’s design to model and simulate a culture at 1:5 scale. This study demonstrated that at an assumed annual cultured meat consumption rate of 10 kg/person, this 300 m3 vessel would be capable of feeding 75,000 people per year at an assumed work rate of one batch per month and 10 runs per year. The non-linear scaling between this approximation and that performed with a 20 m3 vessel in 2014, with yields capable of feeding about 2,500 people annually,17 suggests that a vessel produced at the aforementioned 1,500 m3 scale would be capable of supplying cultured meat for greater than 375,000 people per year. At this rate of production, 889 bioreactors would be capable of providing the current annual meat supply for the entire United States population of 333.3 million people.18 However, with no data yet at that scale, this approximation still lies entirely in the realm of extrapolation.
Air lift bioreactors certainly are capable of providing the throughput necessary for the cultivated meat market, but there remains a significant limiting factor to their utility. Many of cell types utilized in cultured meat products are anchorage-dependent, meaning that they will undergo programmed cell death if not bound to a substrate.14 Furthermore, iPS cells, which are anchorage-independent, form aggregates that can self differentiate as subcultures, destroying the viability of a batch. As a result, suspension-based culturing methods such as stirred batch and air lift bioreactors are unable to culture lab-grown meat without additional aid.9 The primary method used to combat this added layer of complexity includes the introduction of microcarriers to anchorage-dependent cell cultures. These structures provide a surface for the animal proteins to anchor to in order to allow for suspension-based culturing techniques. The optimal material for microcarriers is still yet to be determined, but materials such as edible polymers, hydrogels, and gelatin are currently being explored.9,15
After the cultured meat product is harvested from the bioreactor, downstream processing optimization is still needed. Fortunately, microcarriers made with the aforementioned materials may be able to remain present in the final product, though there are optional processing steps being employed to dislodge the proteins from these structures prior to further processing. This cell dissociation step simply ensures that the microcarriers do not influence the quality of the finalized meat product, and can include both enzymatic and mechanical separation techniques. Whether isolated from the microcarrier or not, the resulting material lacks form and proper composition, making it both visually unappealing and unappetizing for the consumer. At this stage, the produced proteins are able to be mixed with fats, carbohydrates, and other essential minerals to provide the desired flavor profile. Once the mixture is homogenized to the desired degree, the end product can be given its shape through a technique known as scaffolding. Scaffolds provide a three-dimensional physical and biological backbone for the homogenate to adhere and form to, allowing the meat product to take a predetermined form. Currently used scaffolding materials include gelatin, collagen, plant protein, fibrin, decellularized plant matter, edible synthetic polymers, hyaluronic acid, and polysaccharides. Methods for integrating the cultured meat homogenate with these materials include stereolithography, electrospinning, solvent casting, solvent-based extrusion, bio printing, fused deposition modeling, thermal-induction phase separation, selective laser sintering, gas foaming, and freeze drying .This wide variety of viable techniques and materials leaves room for a substantial degree of production optimization as the field of study continues to mature. There is currently no singular method that has been defined as the industry standard, but bioprinting using 3D printing techniques allows for a high degree of control as to the final composition of the end product, making it a highly explored option within the industry.16–18
Competing technologies
Ethical driving forces behind the cultured meat movement include the reduction of carbon emissions, clean water consumption, antibiotic usage, and land dependence of the current meat industry while simultaneously increasing the wellbeing of farmed livestock.12 Many of these issues are already beginning to be addressed in what is being called sustainable meat production.6 Sustainable meat options that are non-cultured include extensive meat, reduced carbon footprint meat, local meat, and organic meat. These means of production are not by definition mutually exclusive, and though they all include compromises can be practiced in parallel to further increase the ethical fidelity of the produced meat product. Extensive meat production leverages land that is not suitable for agricultural development in order to reduce the viable land consumed by traditional farming method. If managed properly, extensive livestock farming is able to aid in the preservation of natural habitats as a result of its non-intrusive nature. Reduced carbon footprint meat is produced with a low dependence on fossil fuels and nonorganic fertilizers, utilizing grazing-based diets and rotational grazing to reduce overall carbon emissions per animal raised. Much of the livestock raised in this manner is also distributed as local meat, minimizing the distance that the meat is transported after processing to further cut down on carbon emissions. Organic production requires the usage of no artificially synthesized fertilizers, pesticides, chemicals, or antibiotics. Furthermore, livestock qualifying as organic must be of an indigenous breed to the location of production, with a maximum stocking rate of 2.4 Livestock Unit/ha. These methods combined are able to begin addressing the issues seen in traditional farming, but they still require substantial compromises. In order to uphold the diversity and quality of livestock life promoted by organic farming, more land must be consumed by farming efforts. Meanwhile, none of the methods mentioned were able to reliably reduce water consumption rates.
Mycoprotein meat alternatives attempt to address all of the aforementioned sustainability concerns, with one primary caveat. Regardless of what is able to be done to enhance taste and texture, mycoprotein solutions are meat alternatives, not meat. That said, with the successful implementation of agricultural waste-based production of mycoprotein, the environmental impact of its manufacturing could be far below that of traditional meat products.19 Under these conditions, overall carbon emissions, land usage, and water usage could be reduced by up to 66.6%, 93.8%, 99.6% respectively when compared, per kilogram protein, to pork.19–21 Unfortunately, this method of cultivation, at an industrial scale, is still in development, and until it is fully implemented mycoprotein development only remains marginally more environmentally friendly than general traditional farming methods. Mycoproteins have also demonstrated cholesterol-lowering effects with consistent consumption that makes them an enticing option for a meat alternative that combats some of the adverse health effects found in traditional red meats.22 With a rise in health-consciousness and vegan dietary trends, mycoprotein is expected to experience notable market growth in both the United States and Europe in the coming decade.23 Projections estimate a compound annual growth rate (CAGR) of 12.6% between 2022 and 2032, surmounting a market valuation of $976 (USD) by 2032.
Market size and growth trends
With competing technologies such as mycoprotein meat alternatives projected to increase in value over the next 10 years, it stands to reason that cultured meat, as well as other sustainable meat production alternatives, are sure to follow. In 2021, the global market share of cultured meat was valued between $129.7 and $163.6 million (USD).7,4 In 2022, there are 31 different companies actively pursuing cultivated meat products, many of which at varying stages of the product development pipeline. Considering the industrial race to bring cultured meat products to the market, projections place the estimated CAGR of cultivated meat as high as 15%, culminating in an estimated global valuation of $450 million (USD) by 2030. Companies within the United States account for 45.2% of those actively pursuing cultivated meat products, but the estimated CAGR of 12.1% 2022 to 2028 within the Asia Pacific region will likely result in a severely decreased market share held by the United States in the near future. Industrial growth projections are promising, but public perception and willingness to adopt lab-grown meat as a viable alternative will ultimately drive the continued development of the market.. A 2021 poll in the United Kingdom indicated that only a third of participants would be willing to try lab-grown meat, implying potential growing pains that could further slow the development of the cultivated meat market as a whole. However, for a food based product such as this, public adoption will be heavily dependent on three factors; safety, eating experience, and ethical implications. These factors will be further addressed in the Discussion section below.
Safety when concerning cultured animal cells intended for human consumption is new territory for regulatory bodies, but the FDA and USDA have established a formal agreement to ensure that it is not overlooked.25 The agreement is designed to outline the collaborative relationship between the two regulatory bodies without creating legally binding obligations. In doing so, the safety of cultured meat products can be ensured until both parties are able to establish their legal jurisdiction and responsibilities in a more detailed manner. These two parties will ensure the safety of these products within the United States, whereas foreign markets will rely on their respective analogs of these parties to do the same. The FDA has also clearly expressed their intent to perform routine inspections of the cell banks, facilities, and cultivated meat products. Such inspections will greatly reduce the probability of potential safety hazards for both the consumer and those involved in production.4
With market interest increasing, the eating experience of cultivated meats has already been put to the test analytically. A study using an electronic tongue system with five distinct “taste sensors” attached to lipid membranes.26 Each of the sensors were calibrated corresponding to one of the five recognized taste sensations using artificial standard solutions: 0.005% lactic acid to calibrate for sour substances, 0.001% quinine hydrochloride to calibrate for bitter substances, 0.8% sucrose to calibrate for sweet (and astringent) substances, 0.4% monosodium glutamate to calibrate for umami substances, and 0.05% sodium chloride to calibrate for salty substances. A flavor solution was then prepared by extracting the flavor from 1 g of minced muscle tissue under standardized conditions. A ratio of the aforementioned flavor profiles were then established using traditionally produced chicken and beef, and compared to their lab-grown equivalents. The data indicated that the cultivated meat demonstrated significantly less bitterness and umami than their traditionally prepared equivalent, indicative of poor perceived flavor when compared to the traditional equivalent. Improvements in flavor can be artificially provided through the use of prescribed seasonings to match the desired meat flavor, but this would ideally be unnecessary. Further cell-line engineering is also able to be performed to better match the desired flavor profile and eliminate the need for such prescribed seasoning methods. Improving the flavor profile of stand-alone cultivated meat will be essential to the larger adoption of this technology.
Ethically, lab-grown meat demonstrates significant advantages over the traditionally prepared alternatives. Considerations used to measure ethics include those used when discussing competing technologies: reduction of carbon emissions, clean water consumption, antibiotic usage, and land dependence of the current meat industry while simultaneously increasing the wellbeing of farmed livestock. Traditionally produced beef products are responsible for about 6.7-8.7 kg CO2 equi/ kg meat protein, making the production of 686 kg of cow protein equivalent to the yearly emissions of a typical passenger vehicle. Cultured meat, when utilizing cyanobacteria hydrolysate as a nutrient and energy source, on the other hand emits between 1.9 and 2.24 kg CO2 equi/ kg meat protein. Even when considering lower emission species such as chicken, cultured meat results in less average emissions, with chicken products being responsible for about 2.2 kg CO2 equi/ kg meat protein. Additionally, lab-grown meat cultivated by these means utilizes 99% less land, and between 82%-96% less water than the alternative production methods. Reducing factors that promote antibiotic resistance is yet another key factor in ethical meat production, as the presence of antibiotic resistant bacteria is shown to be zoonotic when present in livestock for human consumption.27,28 In livestock maintenance, the use of antibiotics helps to maintain yields as well as the health of the heard, but when used as freely as they are in agriculture, antibiotic resistant bacterial strains are guaranteed to emerge.2,27,29 However, since cultivated meats are able to be produced under sterile, controlled conditions, there is no need for the use of antibiotics in any stage of production. Lastly, the cells used to inoculate cultures for cultivated meat production can be harvested without lethally harming the host organism, allowing for animal cell repositories to be made without the need for slaughter. From an environmental perspective, there is no question that the implementation of cultivated meat is more ethical than that which is traditionally produced.
Prospective
Cultured meat products have the potential to take over a large percent of the market share of traditionally prepared meats. Though current means of production are still prohibitively expensive in terms of replacing traditional meat production, the technology is still incredibly young. There is substantial room for cost and resource reduction through the implementation of alternative media and improved yields. With the potential to produce large yields within a fermentation vessel being proven in mathematical models, it is now a matter of funding and application. Funding through government agencies as well as private industry to progress this technology are aiding to meet this need,35 but the approval of further cultured meat products by the FDA and USDA are likely to bring in further investments. From an ethical and practical perspective, this technology has the chance to set a new standard for human food production, and create a historic paradigm shift in the way that humans interact with the world around them. Humans will need to embrace alternative protein sources in the near future in order to keep up with the ever-growing population,9 but the answer to the question of which alternative will garner the most positive human feedback remains to be seen (Figures 1–3) (Table 1).31–45
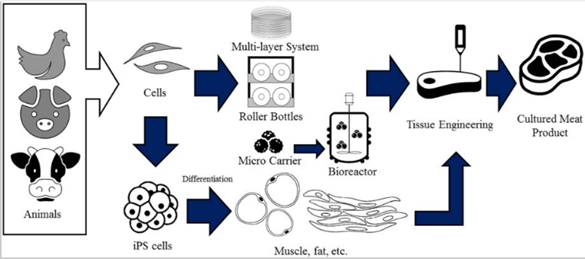
Figure 1 Pictured above is a general production pipeline from animal cell to finalizedcultured meat product.8
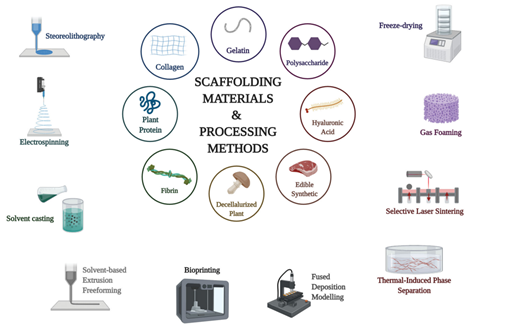
Figure 2 Pictured above are all of the materials and methods currently being explored for use as biological scaffolding.8

Figure 3 The above figure displays the percent of the global market share of each country in the cultured meat industry.5
|
Company |
Country of Origin |
Lab-grown Product |
Website |
|
Balletic Foods |
United States |
Not Available |
https://balleticfoods.com/ |
|
Because Animals |
United States |
Cat and Dog Food |
https://becauseanimals.com/ |
|
Biftek |
United States |
Media for lab-grown meat manufacturers |
https://biftek.co/ |
|
BioBQ |
United States |
Beef |
https://www.biobqing.com/ |
|
BlueNalu |
United States |
Seafood |
https://www.bluenalu.com/ |
|
Bond Pet Foods |
United States |
Chicken for pet food |
https://www.bondpets.com/ |
|
Finless Fodds |
United States |
Seafood |
https://finlessfoods.com/ |
|
GOOD Meat |
United States |
Chicken |
https://www.goodmeat.co/ |
|
Upside Foods |
United States |
Chicken |
https://upsidefoods.com/ |
|
Mission Barns |
United States |
Bacon, sausage, meatballs |
https://missionbarns.com/ |
|
New Age Eats |
United States |
Sausage |
https://newageeats.com/ |
|
Pearlita Foods |
United States |
Oyster |
https://www.pearlitafoods.com/ |
|
Wild Earth |
United States |
Dog food |
https://wildearth.com/ |
|
Wild Type |
United States |
Salmon |
https://www.wildtypefoods.com/ |
|
Aleph Farms |
Israel |
Steak |
https://www.aleph-farms.com/ |
|
BioFood Systems |
Israel |
General meat |
https://www.biofood-systems.com/ |
|
Future Meat Technologies |
Israel |
Chicken |
https://future-meat.com/ |
|
MeaaTech 3D |
Israel |
Beef and poultry |
https://steakholderfoods.com/ |
|
SuperMeat |
Israel |
Chicken |
https://supermeat.com/ |
|
Meatable |
Netherlands |
Beef |
https://meatable.com/ |
|
Mosa Meat |
Singapore |
Seafood |
https://mosameat.com/ |
|
Shiok Meats |
Spain |
General meat |
https://shiokmeats.com/ |
|
Biotech Foods |
Spain |
Omega-3 supplements |
https://www.biotech-foods.com/ |
|
Cubiq Foods |
Spain |
Omega-3 supplements |
https://www.cubiqfoods.com/ |
|
Gourmey |
France |
Poultry |
https://www.gourmey.com/ |
|
Vow |
Australia |
Kangaroo |
https://www.vowfood.com/ |
|
Avant Meats |
China |
Fish |
https://www.avantmeats.com/ |
|
Higher Steaks |
United Kingdom |
Pork |
https://www.highersteaks.com/ |
|
Peace of Meat |
Belgium |
Fat |
https://peace-of-meat.com/ |
|
Clear Meat |
India |
Chicken |
https://www.clearmeat.com/ |
|
Mogale Meat |
South Africa |
Free-roaming livestock and Antelope |
https://mogalemeat.com/ |
Table 1 The above table includes information on all of the cultured meat companies currently active in the world
Source: Cudmore D.5
None.
There are no conflicting interests declared by the authors.

©2023 Krol, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.