Journal of
eISSN: 2572-8466


Review Article Volume 10 Issue 1
UCLA Bioengineering, USA
Correspondence: Bill Tawil, UCLA Bioengineering, USA
Received: February 11, 2023 | Published: February 23, 2023
Citation: Lueptow LM, Tawil B. Breakthroughs in gene therapy: technological progress, current treatments, and future potential. J Appl Biotechnol Bioeng. 2023;10(1):37-43. DOI: 10.15406/jabb.2023.10.00325
In 1973, following the confluence of numerous important discoveries regarding DNA and gene cloning, including the publication of a seminal paper describing the successful transfer of genes into human cells, Friedmann and Roblin proposed the idea of gene therapy for the treatment of human genetic diseases.1–3 It would take almost 30 years for gene therapy to make it into a clinical trial and another 25 years for the first gene therapy treatment to be approved in the U.S.4 The intervening years were filled with several serious setbacks, as well as exciting progress, and currently, there more than 20 cell and gene therapies approved for human use by the U.S. Food and Drug Administration (FDA).4,5
In 1990, Rosenburg and colleagues reported their findings from the very first clinical study using gene therapy in the U.S.6 They extracted tumor-infiltrating lymphocytes from 5 patients with metastatic melanoma, used a retroviral vector to transduce the gene for resistance to neomycin, expanded them in vitro, and then reinfused them back into the patients. This demonstration of safety led to many other clinical studies involving gene therapy. However, several failures in the early 2000s led to major setbacks in the field. One of the biggest failures came when 18-year-old Jesse Gelsinger died after taking part in a clinical trial to treat a minor liver enzyme deficiency using a high dose of adenovirus.7 He suffered severe immunoreactivity to the virus, which led to multiorgan failure and death within days. Another setback came following a clinical trial for X-linked severe combined immunodeficiency (X-SCID) using hematopoietic stem cell gene therapy, which led to several instances of vector-related leukemia.8 While these failures were unfortunate, they provided incredible learning opportunities that opened the door to new approaches and techniques to gene therapy.
The following will outline the basics of gene therapy, mechanisms of current treatments and major technological advances over the years, and the future potential of the gene therapy market.
Techniques in gene delivery: the basics
Simply put, gene therapy is the transfer of genetic material into cells for the purpose of modifying a biological function of those cells, ultimately with the intention of treating a disease.5,9,10 As outlined by the FDA, gene therapies may involve “replacing a disease-causing gene with a healthy copy, inactivating a gene that is functioning improperly, or introducing a new gene to help treat a disease or deficiency”. When cellular function is compromised by a lack of function of a gene, such as in the hemophilia diseases, gene augmentation is used, which involves insertion of a healthy copy of a gene into a cell without replacing the diseased gene (Figure 1a). This results in production of the missing product that is necessary for successful cellular function. When cellular function is compromised due to inappropriate gene expression or overexpression, such as in Huntington’s disease, gene suppression is used, which involves inhibition of the dysfunctional gene via RNA interference (Figure 1b).4 The newest approach is direct gene editing, which involves targeted cleavage of DNA with either homology-directed repair that results in correction of the mutated gene via homologous recombination, or non-homologous end-joining, which results in either gene knock-down or addition the of a functional gene in the presence of an exogenous DNA template insertion (Figure 1c).
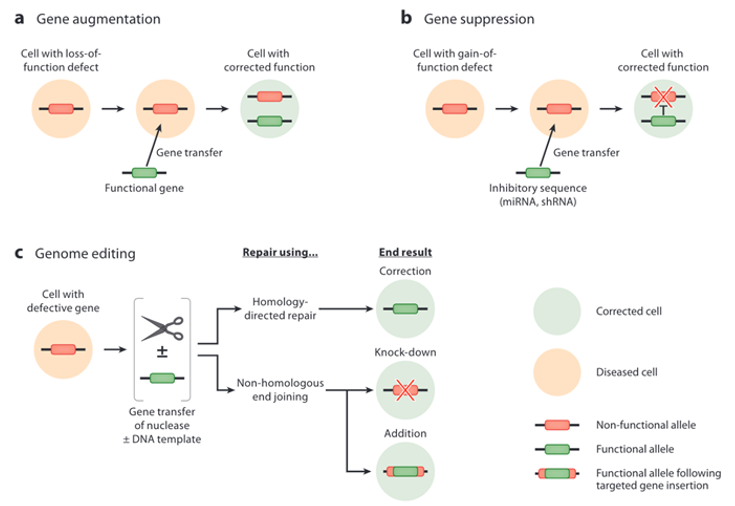
Figure 1 Three main approaches to gene editing.4
Gene therapy can be accomplished in one of three ways: ex vivo, in vivo and in situ.11 In vivo interventions involve administration of the therapeutic directly into the patient, usually systemically into the blood or, in the case of brain diseases, via cerebrospinal fluid (CSF). Administration directly into an organ or specific tissue is referred to as in situ administration; for example, direct injections into the eye for retinal diseases. And finally, many blood diseases or cancers can be treated using ex vivo therapies, which involve removal of target cells from a patient’s body, applying the genetic manipulation, and then returning the altered cells back into the patient.11
One of the most critical factors in gene therapy is deciding how to deliver the genetic payload into the cell.4,12 The choice of vector will determine whether there is integration into the recipient genome and germline transmission, the duration of transgene expression, the likelihood of an immunologic reaction, and the gene size capacity. The vector also determines the cell types and tissues that are transfected. Vectors can be either viral or non-viral, but the primary mode of delivery to date has been via viral vectors. Because ex vivo gene editing is often targeting replicating cells, the genetic material must be able to stably integrate into the genome so that the new gene is replicated in all daughter cells. However, when administering therapeutics in vivo, the targeted cells are primarily postmitotic somatic cells, so different approaches are needed for successful transfection. Furthermore, transduction efficiency is an important factor in achieving a therapeutic effect while also avoiding insertional genotoxicity, and vectors must protect the genetic payload from biological attack or degradation prior to reaching the desired target.10
Techniques in gene delivery: viral vector design
Viruses are a top choice of vector due to their natural proclivity to infect human cells, allowing researchers to leverage various infection mechanisms evolved by viruses over time.12 There are several viruses that have been used as vectors in gene therapy for pre-clinical and clinical studies, and currently 12 FDA approved viral vector-based treatments (Table 1). The ideal vector has a high capacity for the desired transgenes, is replication-deficient, has low likelihood of eliciting an immune response, is non-pathogenic integration, and has a long duration of expression.13–18
|
|
Retrovirus |
Lentivirus |
Herpes Simplex Virus |
Adenovirus |
Adeno-associated Virus (AAV) |
|||
|
Provirus |
ssRNA |
ssRNA |
dsDNA |
dsDNA |
ssDNA |
|||
|
Capacity |
8 kb |
8 kb |
>30 kb |
<8 kb |
<5 kb |
|||
|
Tropism |
Dividing cells only |
Broad |
Broad |
Broad |
Broad |
|||
|
Genome |
Yes |
Yes |
Poor |
Poor |
Poor |
|||
|
integration |
||||||||
|
Inflammatory |
Low |
Low |
High |
High |
Low |
|||
|
potential |
||||||||
|
Duration |
Long |
Long |
Transient |
Transient |
Long |
|||
|
of expression |
||||||||
|
Main |
Persistent gene expression in dividing cells |
Less toxic than retroviruses |
Large packaging capacity |
Efficient transduction in most tissue |
Non-inflammatory; non-pathogenic |
|||
|
Advantage |
||||||||
|
Main |
Potential integration mutagenesis |
Potential integration mutagenesis |
Induces inflammatory response |
Induces inflammatory response |
Small packaging capacity |
|||
|
Disadvantage |
||||||||
|
Indications |
T-cell therapies; immune-deficiencies |
T-cell therapies; neurodegenerative diseases |
Melanoma, solid tumors, other cancers |
Monogenic diseases; vaccines; cancers |
Retinal dystrophies; hemophilia; CNS diseases |
|||
|
FDA Approved Therapeutics |
Yescarta & Tevartus, Kite Pharma; |
Kymriah, Novartis; Breyanzi, BMS; Carvykti, Janssen; Abecma, BMS; Skysona & Zynteglo, Bluebird bio; |
Imlygic, Amgen; |
(various pre-clinical and clinical studies) |
Hemgenix, CSL Behring; Luxturna, Spark Therapeutics; Zolgensma, Novartis |
|||
|
/Companies |
|
|
|
|
||||
Table 1 Commonly used viral vectors for gene therapy
Viral vectors typically have three main components: the protein capsid or envelope, the new genetic material (transgene), and the regulatory cassette. The capsid or envelope encapsulates the genetic material and is critical in determining which cells and tissues the virus is able to infect, known as tropism. The regulatory cassette consists of the other components responsible for expression of the transgene as either an episome, which replicates outside of the host genome, or as chromosomal integration (Figure 2).19
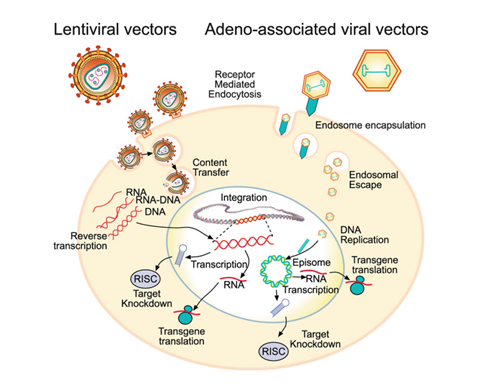
Figure 2 Lentiviral vs AAV-based transfection processes showing genetic integration vs episomal expression.19
Retro- and Lentiviruses
Lentiviruses are a subset of retroviruses that are able to transfect non-dividing cells, whereas retroviruses are only able to transfect mitotically active cells. Retroviruses as a family are spherical, enveloped, RNA viruses around 100 nm in diameter that have a few common genes, gag, pol, and env, which encode capsid and matrix proteins, transcription and integration machinery, and transmembrane and glycoproteins that determine cellular tropism (Figure 3a). Lentiviruses have additional auxiliary genes that increase viral titer and pathogenesis. Retroviruses, including lentiviruses, are the top choice for ex vivo gene therapies, as they are able to integrate into the genome and have sustained expression over time. However, this is also a main drawback of retroviruses, as it places cells at a higher risk for integrational mutagenesis, so extreme caution must be taken in their design.20
The lentivirus HIV-1 (human immunodeficiency virus 1) is the most commonly used and extensively characterized of the lentiviruses. First generation HIV-1 consisted of a three-plasmid system: a transfer plasmid, containing the transgene cassette with all protein coding genes deleted, a packaging plasmid with all other viral genes, and an envelope plasmid with genes from alternate viruses for optimizing target tropism (Figure 3b). With the 3 plasmids, recombination was a big concern, as they were thought capable of generating replication competent viral particles.20 A major advancement came in 1998 when researchers discovered that certain virulence factors (nef, vif, vpr and vpu) were not necessary for viral replication, which led to the second generation of HIV-1 viruses with a significantly decreased risk for viral replication resulting in increased safety.21
Third generation HIV-1 vectors took a big step forward in terms of safety. The tat dependent promoter on the 5’ LTR of the transfer plasmid was replaced with a chimeric CMV promoter, and the packaging plasmid was split into 2, separating rev and POL, resulting in an increased number of recombinations needed in vivo for creation of a replication competent virus (Figure 3b). An additional deletion of the promoter/enhancer elements in the 3’ LTR termed the Self-inactivating (SIN) third generation vector, resulted in loss of the vector’s ability to transcribe full length RNA after integration. An alternate option is to make use of an integrase deficient vector, which prevents any integration into the host genome, but instead recombines to form stable episomes in the host nucleus. While safety is greatly improved, transgene expression will be lost in mitotically-active cells.
In terms of the capsid design, lentiviruses generally do not illicit a humoral immune response, but their glycoprotein envelope provides narrow tropism, with most transfection occurring in CD4+ T-cells. However, pseudotyping, or producing vectors with capsid/envelope proteins from other viruses, has allowed expanded tropism of lentiviral vectors.19–21 Vesicular stomatitis virus glycoprotein G (VSV-G) binds the low-density lipoprotein receptor, which is expressed on most nucleated cells and is commonly used in lentiviral vectors to increase tropism.

Figure 3 Lentiviral vector design of HIV from wild type virus to first, second, and third generation advancements.20
Adeno-associated viruses
AAVs are from the family Parvoviridae and consist of non-enveloped, icosahedral capsid enclosed, single-stranded DNA viruses that are only about 25 nm in size (Figure 4a).20,22 AAVs have a great safety profile, with wide tropism, and stable expression that makes them great vectors for in vivo applications. AAVs encodes only 2 open reading frames, REP, CAP, and AAP, which are flanked by inverted terminal repeat (ITR) sequences (Figure 4b). CAP is translated to capsid proteins VP1-3, while REP is translated to 4 non-structural factors that aid genome replication and virion assembly, and AAP is essential for capsid assembly.20,22,23 All AAVs express 60 VP subunits at a 1:1:10 ratio of VP1:VP2:VP3, and each subunit has 9 variable regions on the capsid that determine the primary tropism and trafficking of the vector, and serves as a prime target for genetic manipulation to enhance tropism and selectivity of transduction.24 An AAV serotype is determined by the binding receptors and tissue tropism, and at least 12 natural serotypes have been identified (Figure 4c).
Aside from capsid design, AAV vectors are assembled in one of three ways: single-stranded (ss), self-complimentary (sc), or split genome (Figure 4b). ssAAVs are generated by removing all of the viral genome except the flanking ITR sequences. The vector is replication incompetent with capacity for ~5 kb transgene, but upon arrival in the nucleus, ssAAVs must undergo second strand synthesis to avoid degradation by the host cell, resulting in low transduction efficiency. scAAVs were created to bypass this step, and contain self-complimentary vector backbones, resulting in significantly increased transduction efficiency. However, the vector transgene capacity is halved, putting significant limitations on the packaging capacity of these vectors.
More recently, a major advancement in vector design has allowed for significant increases in the AAV packaging capacity. The split genome approach takes advantage of the natural tendency of AAVs to undergo homologous recombination of the ITR sequences following transduction into the nucleus (Figure 4b). This approach can utilize 3 or more separate AAV vectors, increasing transgene capacity to 15 kb, though larger viral doses are required for adequate transgene expression.
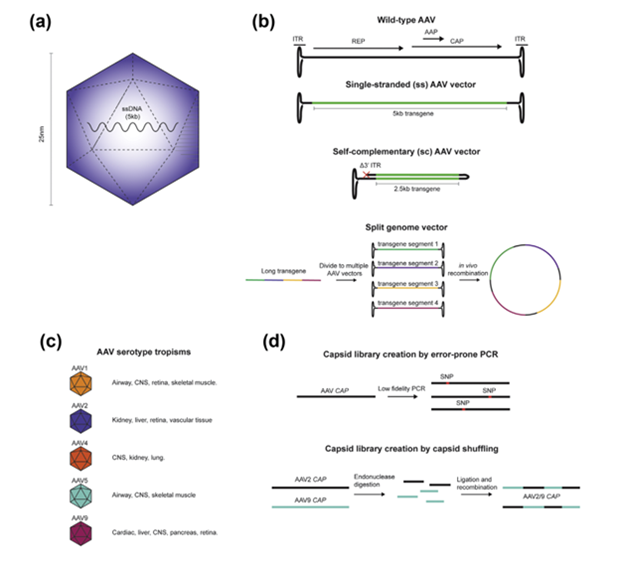
Figure 4 AAV virus (a), vector design (b), naturally occurring serotypes (c), and novel approach to engineering capsids (d).20
While numerous AAV-based gene therapeutics have shown efficacy in vivo, a major drawback of using the naturally occurring capsid serotypes is their proclivity to accumulate in the liver, which can limit tissue targeting and lead to toxicity.17,20,22,23,25 Researchers have developed three main approaches to modify capsid design in hopes of enhancing transduction and immune tolerance: Rational Design, Directed Evolution, and Computer-guided Design (Figure 5). Rational design takes advantage of the known properties of naturally occurring serotypes to design mutants for specific purposes.25 For example, AAV2.5 was generated by engrafting residues from AAV1 into the AAV2 capsid, resulting in enhanced transduction into muscle tissue for the treatment of Duchenne muscular dystrophy. Directed evolution takes advantage of random recombination and low-fidelity PCR (Figure 4d) to generate libraries that can be screened with high-throughput methods for novel phenotypes. Computer-guided design takes advantage of DNA sequences and phylogenetic analysis to construct potential AAV capsid library that can then be tested.
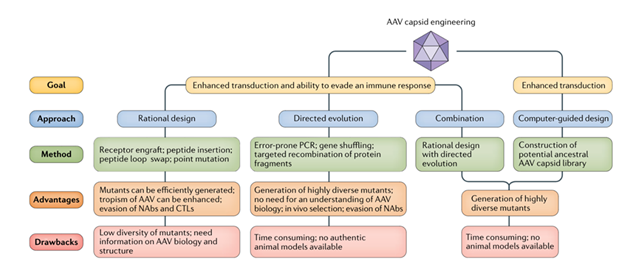
Figure 5 Approaches to AAV capsid engineering.24
Herpes simplex viruses
Herpesvirus (HSV) is an enveloped virus with a genome of more than 150 kb in length that encodes for almost 90 genes.26 Half of the genes are nonessential and can be eliminated, allowing for large transgene insertions. There are eight HSVs, all of which can infect and cause human disease.27 HSVs have broad tropism, especially neurons, and the capacity for delivery of larger transgenes, while also avoiding insertional mutagenesis.28 However, HSVs can go latent and lead to random reactivation and cytotoxicity Proteins expressed on and in HSVs also have been shown to cause inflammation and cytotoxicity.29
To avoid these issues, one specific modification has been developed using HSV amplicons, which are stripped down HSV vectors that lack protein coding genes and rely on helper viruses for replication and packaging (Figure 6).29,30 However, this method makes production and delivery at high enough titers challenging without cross-contamination and cytotoxicity. Newer generations of replication-competent HSV vectors that are able to avoid going latent and are being used in oncolytic therapies and vaccines.28 These are attenuated versions of the native HSV virus, which contains genes necessary for replication in vitro, but cannot replicate in vivo.26
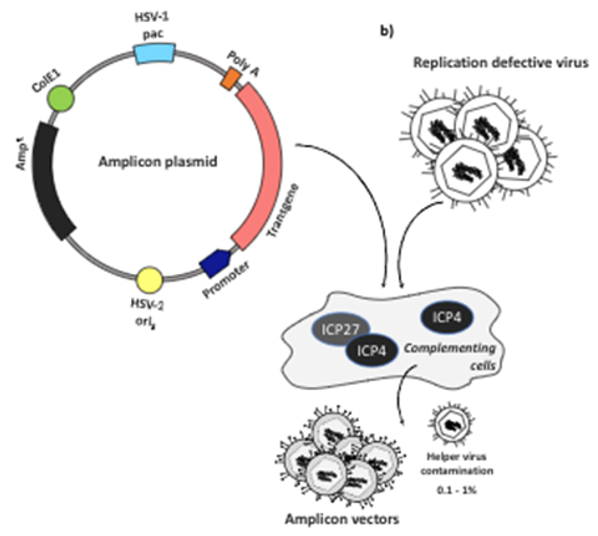
Figure 6 Design and use of HSV amplicons for gene therapy.29
Techniques in gene delivery: non-viral vector designs
While many strides have been made in the development of safer viral vectors, many challenges remain. Non-viral vectors offer an opportunity to circumvent the cytotoxicity, immunogenicity, and mutagenesis concerns across the various viral vectors.31,32 While the majority of clinical trials have utilized viral vectors, non-viral technologies is a fast-paced area of research and clinical trials are expanding. Figure 7 outlines many of the materials currently being used to develop new vehicles for gene delivery. The following will provide a brief overview of a few of the most commonly used materials.
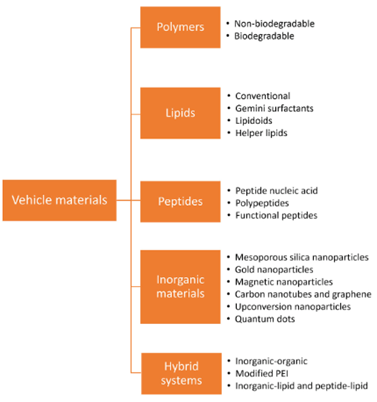
Figure 7 Materials used in non-viral vector design.31
Polymers are large, positively charged complexes that bind negatively charged genetic material and form a polyplex. The positively charged nature of the polyplex facilitates crossing the cell membrane and integration into the cytoplasm or nucleus.31,32 They can be natural (e.g., gelatin, cationic cellulose, cationic dextran, etc) or synthetic. One example of a synthetic is polyethylenimine (PEI), which has high transfection efficiency and when linked to a fluorophore provides protection from enzymatic degradation, has shown high transfection efficiency and low cytotoxicity.16 However, PEI has poor specificity and can accumulate in cells over time causing cytotoxicity.
Lipid-based vectors are commonly used for gene delivery. Lipids are also positively charged and have electrostatic interactions with anionic phosphate groups of the nucleic acids. Because of their tail structures, lipids often self-assemble into liposomes, solid lipid nanoparticles, or lipid emulsions. One major upside is that lipids are bio-degradable, making them less toxic, and can carry hydrophilic or hydrophobic substances.
Lipid nanoparticles (LPNs) have been used for decades to delivery drugs in vivo, but application to gene therapy was limited due to size constraints. However, in 2018 Onpattro from Alnylam Pharmaceuticals became the first FDA-approved siRNA treatment. They carefully modified the LPNs with ionizable cationic lipids that are positively charged at acidic pHs but neutral at physiological pH, which allowed encapsulation in vitro without in vivo cytotoxicity of a highly charged particle. They incorporated polyethylene glycol (PEG)-lipids to increase the LPN size range, but used PEGs with short C14acyl chains, to encourage delivery into the cell.33
Peptides are short chains of amino acid residues anywhere from 2-10 amino acids in length. They are biodegradable and biocompatible and can be easily designed to interact with nucleic acids and form complexes to facilitate delivery. The interaction can either be via conjugation or electrostatic forces that create the peptide nucleic acids (PNAs). The PNAs are linked via covalent bonds, resulting in highly stable, uncharged complexes that are able to avoid nuclease degradation. However, this may result in lower transfection efficiency, and modifications can be made to produce peptide dendrimers that have positively charged groups to enhance ability to pass through the cellular membrane.31,32
A number of inorganic materials have been used to create nanoparticles for delivery of genetic material, including metallic compounds, quantum dots, and carbon nanotubes, among others.31,32,34 Inorganic materials tend to be more stable, with high biocompatibility and negligible cytotoxicity. Metallic-based nanoparticles, including gold and silver, have especially low toxicity, are easily fabricated and can be modified for more specific targeting. Quantum dots are made from semiconductor materials and have the advantage of being used as fluorescent probes, which allows easy tracking when assessing distribution and transfection. While other inorganic vectors depend mostly on endocytosis to enter the cell, carbon nanotubes are able to pass through cell membranes independently. They consist of sheets of graphene that can range in size from nanometers to microns, which has the benefit of large carrying capacity.32
CAR-T and CRISPR therapies
Currently, the use of chimeric antigen receptor (CAR)-T cell therapy has been one of the fastest growing areas of gene therapy for use in cancer. The basic idea is that a patient’s immune cells are removed (generally T cells) and genetically reprogrammed to express CARs, which are engineered synthetic receptors that recognize antigens expressed on certain cancer cells.35,36 CAR T cells are able to recognize and be activated by simple antigen recognition on a tumor cell, avoiding the traditional cascade of immune events needed to activate killer T cells.35 While there has been great success in these treatments, there are also a number of limitations, including antigen escape, where there is loss of the targeted antigen following treatment. There can also be potential off-target effects, so choice of antigen target is crucial in minimizing side effects. Finally, CAR-T therapies have had limited success with solid tumors, as they have limited ability to infiltrate and thrive in the tumor microenvironment.
Techniques using direct genome editing have seen the greatest technological advancements in the last 10 years. Specifically, the discovery of CRISPR (clustered regularly interspaced short palindromic repeats)-CAS system was described by scientists investigating bacterial immune defenses.37,38 Briefly, guide RNA directs Cas nucleases to a specific target sequence on the DNA where there is a double-stranded break, which allows for a gene deletion or insertion. The great advantage of CRISPR is that it uses a short sequence of RNA to guide the complex, whereas previous approaches relied on difficult protein-DNA interactions. The guide RNA is also very easy to design and the approach can be done in vivo. Currently the biggest issues facing the advancement of CRISPR for medicinal use is risk of off-target effects (i.e., editing accuracy) and the ability to precisely edit DNA with homology directed repair to obtain the desired outcome. However, progress continues to be made on both fronts, and in 2021, the FDA approved the first CRISPR based clinical trial for Sickle Cell Disease by a group out of Berkely.39 In 2022, two other companies have received acceptance of their applications to the FDA for approval for treating blood disorders using CRISPR, and numerous preclinical and clinical trials are underway using CRISPR technologies for various indications, including for amyloidosis, solid tumors, retinopathies, and hemoglobinopathies, and more.37,40
Clinical opportunities and future obstacles
Within the FDA, the Center for Biologics Evaluation and Research (CBER) regulates gene therapy products and devices, providing regulatory oversight of clinical studies, as well as companies and manufacturers in product development.41 There are currently 12 FDA approved gene therapy products (Table 2), covering a wide range of indications. While there may be relatively few approved therapeutics, there are hundreds of clinical trials currently being conducted across Phase I, II, and III trials (Figure 8), and the forecasted growth in the market is expected to soar over $1 trillion by 2025. Moving forward, there are several important challenges with the currently available gene therapy technologies that can be improved upon. Recent market analysis has identified 5 areas that require improvement moving forward: capsid design, vector design, new types of cargo, improved manufacturing processes, and improved pretreatment and conditioning regimens.42,43
|
Therapeutic |
Manufacturer |
Indication |
Mechanism of Action |
|
Abecma (idecabtagene vicleucel)44 |
Celgene Corp, Bristol Myers Squibb |
Relapsed or refractory multiple myeloma |
ex vivo CAR-T cell therapy via lentiviral vector |
|
Breyanzi (lisocabtagene maraleucel)45 |
Juno Therapeutics, Bristol Myers Squibb |
Large B-cell lymphoma |
ex vivo CAR-T cell therapy via lentiviral vector |
|
Carvykti (ciltacabtagene autoleucel)46 |
Janssen Biotech |
Relapsed or refractory multiple myeloma |
ex vivo CAR-T cell therapy via lentiviral vector |
|
Hemgenix (etranacogene dezaparvovec-drlb)47 |
CSL Behring |
Hemophelia B |
in vivo AAV5 gene augmentation |
|
Imlygic (talimogene laherparepvec)48 |
Amgen |
Melanoma |
in situ oncolytic treatment via HSV-1 |
|
Kymriah (tisagenlecleucel)49 |
Novartis |
Relapsed or refractory follicular lymphoma |
ex vivo CAR-T cell therapy via lentiviral vector |
|
Luxtrurna (voretigene neparvovec-rzyl)50 |
Spark Therapeutics |
RPE65-mutation associated retinal dystrophy |
in situ AAV2 gene augmentation |
|
Skysona (elivaldogene autotemcel)51 |
bluebird bio |
Active cerebral adrenoleukodystrophy (CALD) |
ex vivo gene augmentation via lentiviral vector |
|
Tecartus (brexucabtagene autoleucel)52 |
Kite Pharma |
Relapsed or refractory mantle cell lymphoma (MCL) |
ex vivo CAR-T cell therapy via retroviral vector |
|
Yescarta (axicabtagene ciloleucel)53 |
Kite Pharma |
Large B-cell lymphoma |
ex vivo CAR-T cell therapy via retroviral vector |
|
Zyntelgo (betibeglogene autotemcel)54 |
bluebird bio |
Beta-thalassemia |
ex vivo gene augmentation via lentiviral vector |
|
Zolgensma (onasemnogene abeparvovec)55 |
Novartis Gene Therapies |
Spinal Muscular Atrophy (Type I) |
in vivo AAV9 gene augmentation |
Table 2 Current FDA approved gene therapies

Figure 8 Number of clinical trials for various gene therapies.42
As discussed above, capsid design is critical for cellular tropism and transfection efficiency, but capsids are also critical for stabilization during the manufacturing, storage, and distribution process. Researchers are pivoting to more carefully engineered capsids to overcome many of these issues moving forward. Vector design is also critical, for many of the reasons discussed above. Researchers are continuing to optimize vector engineering to reduce immunogenicity and improve transgene expression. Researchers have also begun experimenting with immunosuppressive treatments to reduce the immune system’s interference with the vector administration, thus potentially allowing repeat treatments or treatments in patients with pre-existing immunity to the virus.
Critically, many companies are making big investments into optimizing manufacturing processes and capacities. Original manufacturing processes were developed in academic labs and not optimized for large-scale production. One major issue is the accidental production of empty capsids that can taint preparations, requiring administration of higher doses, and methods are being introduced to filter out these empty capsids. Companies like Pfizer are also investing millions to build additional manufacturing facilities to expand production capabilities.
Since its inception many decades ago, gene therapy has provided as much disappointment as excitement. In the last decade, much progress has been made in advancing gene therapy delivery and gene editing technology, building on those lessons learned over time. While we have entered an exciting new era in gene therapy, many of the approved therapeutics still have significant barriers to overcome. Many treatments are considered a last line of defense, due to their safety profiles and potential for off target effects. Due to generated immune responses, many vectors can only be given once, limiting their usefulness in the long-term. However, advancements are occurring at a breakneck pace, and as with the last few decades, the challenges and setbacks will inform future progress.
None.
There are no conflicting interests declared by the authors.

©2023 Lueptow, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.