Journal of
eISSN: 2572-8466


Review Article Volume 9 Issue 2
1Department of Biotechnology & Bioinformatics, California State University, USA
2Department of Biomedical Engineering, University of California Los Angeles, USA
Correspondence: Bill Tawil, Department of Biomedical Engineering, University of California Los Angeles, 420 Westwood Plaza, Room 5121Engineering V. P.O. Box 951600, Los Angeles, CA 90095-1600, USA, Fax (310) 794-5956
Received: February 13, 2022 | Published: April 19, 2022
Citation: Debich M, Bill T. Biotechnology and stem cell technology overview. J Appl Biotechnol Bioeng. 2022;9(2):57-60 DOI: 10.15406/jabb.2022.09.00285
animal biotechnology, stem cell, recombinant DNA, replication, translation
In the 21st century, Humanity witnessed a remarkable growth in scientific discoveries and inventions. This scientific revolution is affiliated with many sectors such as biology, chemistry and computer science.1 This scientific progress is due to the challenges that humanity faced in order to improve life quality and save lives of peoples all over the globe.2 For instance, the recent COVID-19 pandemic that threaten the humanity existence. The virus spread in only 20 days, it infected more than 80 million persons and lead to more than 1.6 million deaths.3 Science and specifically Biotechnology had to overcome this obstacle to find a vaccine for this airborne virus threating humanity. In less than a year, Biotechnological laboratories were able to provide Covid vaccines.4 Biotechnology is an interesting scientific sector that rises many questions about its definition, history of development and application.
Definition of biotechnology
Karl Bayer defines Biotechnology as we know nowadays as an interdisciplinary and intradisciplinary collaboration of scientists from various fields. Furthermore, the key disciplines are biological sciences, informatics, biochemistry, mathematics and engineering. The development and the progress of these fields lead to a biotechnological revolution. For example, molecular biotechnology has stimulated biotechnology in particular by the discovery of DNA molecule structure and behavior which lead later on to an understanding and establishment of a new discipline called genomics that focuses on gene sequencing and cloning. In addition, the intensive growing capabilities of computer sciences allowed biotechnology to shift to another level which manifests on the scientists’ methods of storing, analyzing and interpreting biological data.5 Computer sciences established a new sub-biological sector called bioinformatics which caused scientists to modify their methodology from a hypothesis driven to a data driven investigation using statistical modeling.6
Branches
Observers and specialists believe that the history of biotechnology should be characterized by its timeline and applications or branches.7,8 Biotechnology is composed of 4 branches, Red biotechnology, Green biotechnology, Blue biotechnology and White biotechnology. Red biotechnology or as referred as medical biotechnology, it mainly focuses on the health care field including pharmaceuticals, medicine , medical devices and any other technology affiliated with curing and treating patients. In addition, green biotechnology includes agriculture and veterinary medicine such GMO (genetically modified organisms) plants and animals able to resist diseases and extreme weather changes. Moreover, blue biotechnology is simply marine biotechnology which studies life in marine and aquatic environments. Finally, white biotechnology which compasses researches on developing new industrial processes such as the production of environment friendly fuels.
Timeline
Brian Colwell divided the history of biotechnology into 3 distinct phases; ancient biotechnology, classic biotechnology and modern biotechnology.
Phase 1
Ancient biotechnology (pre-1800) has been used by human since dawn of civilization. It started when human domesticated corps and wild animals. In the early 7000 BCE researchers discovered that yeast, one of the oldest bacteria was used by Sumerians and Babylonians to ferment beer and to make bread. At the end of ancient phase, in 1796 Edward Jenner inoculated a young boy with a matter collected from young dairymaid infected with cowpox and injected the same young boy with smallpox matter.9 The young boy exhibited some side effects such as fever and loss of appetite after 9 days the young boy recovered. As a result, Edward Jenny discovered a vaccine that protects from smallpox.
Phase 2
Classic biotechnology is the era from 1800 till 1945. In 1855 Escherichia (E-coli) bacterium is discovered. One of the most discoveries that tailored the future of biotechnology. Nowadays, its used to mass produce proteins such as insulin. Scientists still use E-coli to research and analyze recombinant DNA. In 1928, Alexander Fleming discovered the first antibiotic in the world ‘Penicillin’ the from the mold Penicillium notatum. Since Penicillin is able to fight infectious diseases, Pfizer mass produced the latter antibiotic during World War II which was a decisive factor that lead to the allies victory.
Phase 3
The third phase is modern biotechnology. Two major discoveries that shifted the biotechnology to another level where genetic engineering has played an important role. The first discovery was made by Crick and Watson in 1953. They were able to illustrate the shape and the composition of DNA molecules. The second discovery made by Cohen and Boyer who invented and developed the process of producing recombinant DNA. They introduced a foreign sequence of DNA to E-Coli plasmid then transferred those plasmids to other E-coli bacteria (genetic engineering). In 1982, thanks to Watson, Crick, Cohen and Boyer work, FDA approved the first biotechnological drug (Insulin). Genentech and Eli Lilly developed the artificial insulin using genetically modified bacteria.7 In 1983, scientists were able to replicate a specific DNA sequence in vitro with a technique called polymerase chain reaction (PCR). This technique uses a series of heating and reheating at various specific temperatures and also uses specific enzymes such as Taq polymerase which is found in bacteria able to live in high temperatures.10 Kary Mullis successfully developed PCR, thanks to DNA polymerase (involved in DNA replication) discovery in 1955. In Conclusion, I personally believe, that biotechnological applications are not limited to the ones mentioned above. In my opinion the discovery of DNA gave a huge boost to our understanding to the process of replication, translation and translation, from nucleic acid to amino acid. However, without the work done by scientists in any historical phase, we will not be able to benefit humanity and ensure quality of life for the upcoming generations. Recently, biotechnological discoveries have accelerated and elaborated in many segments.
Market size
One of the biotechnology segments or applications that attracted my attention is the Stem Cell technology. The Stem Cell market has been growing remarkably in the past decade with an annual growth rate of 25.5% from 2015 to 2022 and with an expected market global size of US$297 billion by 2022. Graph 1 shows the growth of stem cell market from 2013 to 2024 in the world.11 The market size is decided by stem cells applications which is divided in two major sectors. The first sector is stem cell research which provides a tremendous boost to the second sector. The second sector focuses majorly on clinical application which includes tissue engineering and immunohistochemistry and drug development.
Leading companies
If we focus on the year of 2015, we can see many leading companies all over the world according to Graph 2. For instance, in the Northern America, the key operating companies are Osiris and Neural-Stem Inc. In addition, in the United States, both public and privet sectors showed interest in Stem technology by funding some companies. For example, Kiadis Pharma B.V has collected more than US$13 million to finance the production of ATIR-101 which is an immunosuppressant anticancer drug supported by venture capitalists such as Alta partners. Moreover, the public sector expresses their interest in Stem Cell technology.12 California Institute for Regenerative Medicine (CIRM) has funded ViaCyte with more than US$ 16 million to develop a stem cell therapy for diabetes. Not only does CIRM contribute but also the U.S Department of Health and Human Service’s Biomedical Advanced Research and Development Authority (BARDA) has offered US$ 106 million for preclinical and clinical studies of cell therapy for thermal wounds and radiation injuries (Graph 3).
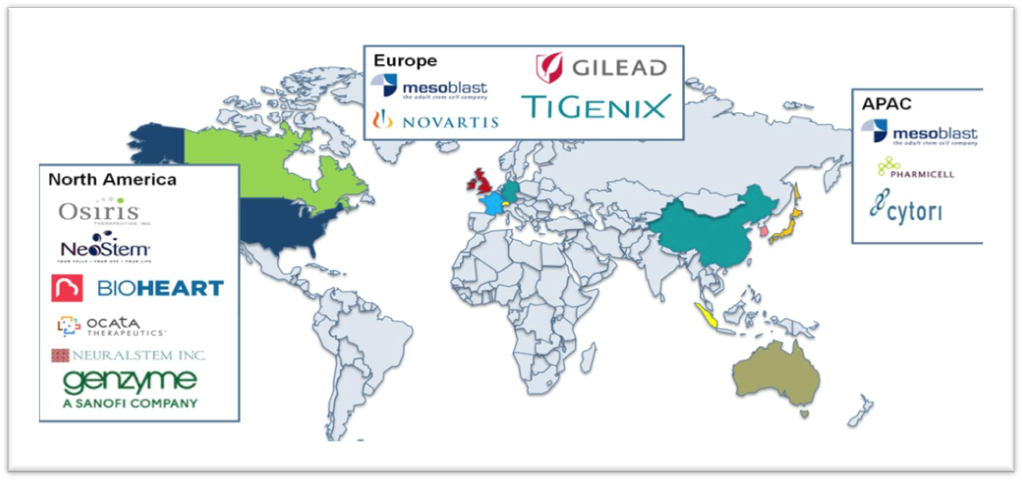
Graph 2 Leading companies in the stem cell therapy market all over the globe, 2015.12
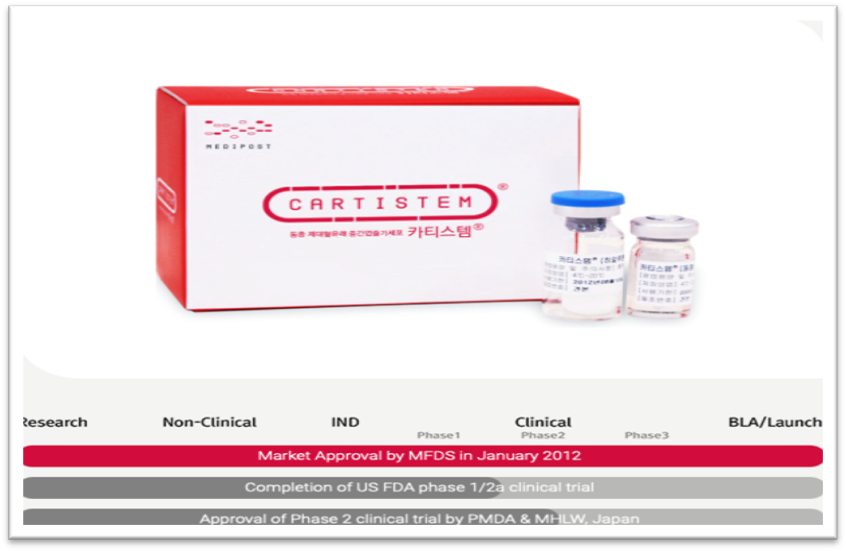
Graph 3 CARTISTEM approval status in South Korea, Japan and The United States.17
Stem cell potential
Stem Cell technology has developed and expanded because of the intrinsic properties of stem cells. Karla and Tomar define stem cells as cells capable of self- renewal and differentiation into multiple specialized cell types. In addition, stem Cells are responsible for replacing and recovering damaged tissue cells. Moreover, they are capable of developing into a wide range of specific types of functional cells to form a tissue such as liver or neurons.13 The differentiation process requires activation or silencing specific genes and intervention of certain molecules or growth factor to initiate such process.14 Stem Cells are classified based on their potency. For instance, embryonic stem cells which are derived from ICM (inner cell mass) which are pluripotent, able to differentiate to all cell type to form the human body.15 In 2006, scientists were able to genetically reprogram differentiated cells into cells similar to embryonic stem cells called iPSCs (induced pluripotent stem cells). Another type of stem cells is Totipotent which are the first few cells formed from division of a zygote. Additionally, multipotent stem cells which have the ability to differentiate into related family of cells. Therefore, there are two major categories of stem cells. Embryonic stem cells which are pluripotent stem cells and adult stem cells which includes undifferentiated totipotent and multipotent stem cells after embryonic development. For example, mesenchymal stem cells that form cartilage and connective tissue.
Medicine derived from stem cell technology
The ability of self-renewing and regenerating damaged tissue by embryonic stem cells and adult stem cells make them good candidates for a new generation of medicine called regenerative medicine which includes the injection of stem cells (stem cell therapy).16 For example, embryonic stem cells and iPSCs have a great potential to replenish damaged and unfunctional beta pancreatic cells that are responsible for synthesizing and secreting insulin. Regeneration of such type of cells presents a permanent cure for a chronic disease such as diabetes.13 Another example of regenerative medicine is CARTISTEM which is composed of mesenchymal cells derived from allogenic umbilical cord blood stem cells.17 CARTISTEM targets patient with Osteoarthritis which is characterized with knee cartilage defects caused by degeneration or repetitive trauma especially for elderly patients. The manufacturer of this drug is MEDIPOST which is a South Korean company specialized in stem cell technology.
CARTISTEM was approved by the South Korean Ministry of Food and Drug Safety (MFDS). However, the drug still not approved by the FDA but just completed phase 2a of clinical trials in the United State. Also, the Japanese authorities have approved phase 2 clinical trials. This product was released in the South Korean market in 2012. In 2018, sales volume of CARTISTEM increased by 40% with a record of 1,100 vials and exceeded US$ 8 million of revenues. In conclusion, I believe that even though the remarkable growth of stem cell market and the various applications from research to therapy, we need to focus more on the ethical side. We need to address ethical concerns surrounding the creation and utilization of embryonic stem cells. Should it be regulated by laws and restrictions?
Tissue engineering
According to the information mentioned above, the market size of stem cell technology reflects the importance of such a field that establishes a new generation of medicine that uses the human cells as raw materials to cure disease or even tissue engineer transplantable organs to save people lives who suffers from vital organ failure.
In my opinion, the availability of artificial organs will benefit many sectors. First, patients will no longer have to be placed in waiting lists to wait for a donor. In addition, as my personal experience, I have a friend who received a kidney from a donor. All his immediate family volunteered to donate. However, the medical stuff decided to go with his mother because they have some similarities such as blood type. Not only that but also, he had to commit to a very strict treatment and therapy to ensure the success of the transplant. Therefore, the survival of a graft is not 100% guaranteed. Imagine if a patient receives a kidney engineered from its own stem cells. This technology offers hope to many patients and saves them time. In these types of situations, time is a very crucial factor, as long as the time passes, the patient’s chances of survival diminish. Tissue engineering has an economic impact, it can tremendously save money to the public. For Example, Dialysis sessions cost per patient US$72,000 per year which means US$ 500 per session and the federal government covers 80% of the cost.18,19
Modelling diseases
Another future application is modelling diseases. This application consists of utilizing iPSCs stem cells derived from a diseased tissue to grow them invitro to comprehend the behavior of healthy cells in presence of abnormal cells. In other words, visualize the pathway of any disease and decide if it is caused by a genetical mutation then sequence the gene responsible for such mutation. Moreover, modeling diseases will ameliorate pharmaceutical testing through applying a potential drug directly on diseased cells avoiding delivery barriers that could minimize the efficacy. I, personally, believe, modeling diseases will save a lot of time concerning the regulatory process of approval. Determining the safe dosage or the side effects could be expected ahead of time without testing the product directly on patients or volunteers. Furthermore, I expect that the FDA will establish a new office specialized on Stem cells technology and reconsider the approval phases from preclinical to clinical time frame wise.
I am sure that stem cells exploitation has tremendously benefited humanity in various sectors by establishing a new type of employment and offering a regenerative medication system. These Stem cells comes from human who need to be asked for permission to use their tissue wether it is healthy or diseased. Here, many ethical questions rise especially if there is a profit made through exploiting someone’s stem cells either for research or therapeutic purposes.
Obstacles facing stem cell technology
Recently, in October 4th of this year, a law suit has been filed against Thermo Fisher Scientific for unpermitted usage of cancerous tissue from an old lady and profiting out of it.20 Brief, the tumor tissue taken from the old lady in 1959 became the first achievement in modern medicine technology. Her cells were cultured and used to develop the polio vaccine, genetic mapping and even COVID-19 vaccines. Those cells are unique since they are able to survive and thrive in laboratories. This exceptional characteristic made it possible to cultivate her cells indefinitely which gives scientist the advantage of performing studies using identical cells. Thermo Fisher Scientific has generated billions of dollars of annual revenues thanks to the old lady’s stem cells. Thermo Fisher Scientific is a gigantic company that have many branches all over the world.21 Therefore, I certainly believe that they have a prestigious legal team that could prevent this bioethical scandal by investigating the source of those cells to prevent such lawsuit.
In 2001, President George W. Bush signed federal law that banned research on embryonic stem cells claiming such technology destroys embryos in order to extract stem cells.22 The scientific community has lost more than US$ 170 million of the federal funding available for research on stem cells derived from human embryos.23 In my opinion, such law held back the research development and evaded an opportunity to better understand factors and genetical signals that initiate the process of transformation from unspecialized stem cell to specialized ones.
In conclusion, laws and regulations have the purpose of identifying the source stem cells and the goal of performing experiences that should be only and mainly under the umbrella of benefiting humanity. Laws and regulation are established to organize the field and protect the rights of both costumers and scientists. Hopefully, in the near future will see the first tissue engineered heart ready to be transplanted and obtain a better comprehension of the pandemic of the current century (cancer) that caused pain and sorrow to many people all around the globe.
None.
The authors state that there is no conflict of interest.
None.

©2022 Debich, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.