Journal of
eISSN: 2572-8466


Review Article Volume 3 Issue 5
Department of Botany, University of Delhi, India
Correspondence: Lokendra Singh, Department of Botany, D.S.B. Campus, Kumaun University, Nainital-263 002, Uttarakhand, India
Received: June 01, 2017 | Published: August 29, 2017
Citation: Singh L. Biodegradation of synthetic dyes: a mycoremediation approach for degradation/decolourization of textile dyes and effluents. J Appl Biotechnol Bioeng. 2017;3(5):430-435. DOI: 10.15406/jabb.2017.03.00081
Present review highlights the role of fungi in biodegradation/decolourization of textile dyes and effluents. Though, fungi and bacteria both are the principal decomposer of xenobiotics in the environment but fungi are superior to bacteria in dyes degradation/decolourization due to their superiority in the production of dyes degrading enzymes and as absorbent. The present study focused on the achievements and advancement on the fungal degradation/decolourization of textiles dyes and effluents by the application of potent fungal strains. The biodegradation through fungi or myco-remediation of textile dyes and effluents using potential fungi is comparatively cheap and environmentally safe process, to decompose or mineralize the hardly or less degrading compounds of synthetic dyes. Fungi play an important role to degrade/decolourize the synthetic dyes through enzymes as well as by the absorption, adsorption and accumulation of colourants from the effluents.
Keywords: biodegradation, myco-remediation, accumulation, textile dyes, effluents, fungi, fungal enzymes, peroxidase, ligninase
TOC, total organic carbon; BOD, biological oxygen demand; COD, chemical oxygen demand; PAHs, polycyclic aromatic hydrocarbons; RBBR, remazol brilliant blue R; LiP, lignin peroxidase; MnP, manganese-dependent peroxidase; Dyp, dye degrading peroxidase
Rapid industrialization, developmental processes or modernization has given rise some unwanted toxic elements into the atmosphere. These elements are accumulated in the biosphere up to toxic level and creating problems in the environment, by destroying the natural ecosystem. For example, a measurable quantity of synthetic dyes as textile discharges or effluents from these industries are coming out directly to natural water bodies (after the dying processes). These hazardous effluents are being settled to water bodies (directly and indirectly) that finally contaminating to the water and soil. Textile dyes and effluents from the dying processes has adverse impacts on the water quality in terms of total organic carbon (TOC), biological oxygen demand (BOD), chemical oxygen demand (COD), colour, pH and presence of recalcitrant synthetic compounds, such as azo-dyes and heavy metals.1,2 The water polluted by the textile industry discharges has received an increased attention for several decades, to set up the strategies to solve the present problem.3 Different types of synthetic dyes and pigments are used worldwide for the coloring processes in textile and allied industries. Inefficiency in dying process, poor handling of spent effluent and insufficient treatment of wastes from dyestuff industries lead to dye contamination in soil and natural water bodies. Untreated textile effluents may contain a large number of heavy metals like, Cd, Cr, Co, Cu, Hg, Ni, Mg, Fe and Mn that are highly toxic, mutagenic and carcinogenic. The pollutants, aggravated by the presence of free chlorine and toxic heavy metals, cause rapid depletion of dissolved oxygen, leading to “Oxygen Sag” in the receiving water.4,5 The textile industries constitute a vast majority of the dyes which are discharged in the wastewaters. Over 100,000 commercial dyes exist and more than 7×105 tons of dyestuff is produced annually, of which 1-1.5×105 tons is released into the wastewaters. These synthetic dyes and their effluents are recalcitrant to microbial degradation and causing problems in the usual biological degradation. Among the synthetic dyes, azo dyes, characterized by the presence of one or more azo groups (-N=N-), and anthrax-quinonic dyes represent the largest and most versatile group of dyes.6–9 The synthetic dyes with azo group (e.g. monoazo, diazo, triazo and polyazo), represent the largest class of organic colorants i.e. account for 60-70 % of the total used synthetic dyes. Moreover, dyes have become a health hazard as many of them and/or their breakdown products have been found to be toxic and potentially carcinogenic.4,10,11
Microorganisms are able to degrade synthetic dyes to non-coloured compounds or even mineralize them completely under certain environmental conditions Bioremediation is one of the most effective and successful cleaning techniques for the removal of toxicants from polluted environments.12–14 The microbial degradation of textile dyes has been reported using different microorganisms including bacteria, yeasts and filamentous fungi.3,15,16 The biodegradation studies on the dyes degradation by using fungi Phanerochaete chrysosporium, Bjerkandera adusta, Trametes versicolor, Phlebia radiata and Pleurotus spp. have been shown the production of enzyme laccase that was found highly related to lignin and dyes degradation.17–21 These fungi are found potent much to degrade a broad spectrum of structurally diverse group of dyes. Heinfling et al.18 reported that Bjerkandera adusta and T. versicolor are able to remove 95% of HRB 8 dye within four days. Due to the fungi oxidative mechanisms it is possible to avoid the formation of hazardous anilines, formed by reductive cleavage of the azo dyes, by other microorganisms such as bacteria.22 The interest in the fungal degradation/decolourization of textile dyes is mainly due to the expression of some non-specific extracellular enzymes, as the ligninolytic peroxidases, that have been implicated in the degradation of synthetic dyes.23,24 White-rot fungi produce several enzymes that have been related to their ability to degrade natural polymers, such as lignin and cellulose, but can also degrade different synthetic chemicals, usually recalcitrant to biodegradation.25,26 The promising results obtained with this ligninolytic fungus, lead to the study of the potentialities of other species of ligninolytic basidiomycetes. The role of fungi in the wastewater treatment has been proved them to be suitable and effective for dyes degradation/decolourization and the removal of colourants from the textile effluents. Dyes and pigments are being removed by these fungi either in living or dead form through bio-sorption, bio-degradation, bio-accumulation and enzymatic mineralization, using lignin peroxidase, manganese peroxidase, manganese independent peroxidase and laccase.27–30 Fungi has been shifted the research interest for the bio-treatment of wastewater ingredients such as metals, inorganic nutrients and organic compounds. Physico-chemical treatment processes, such as coagulation, precipitation, filtration, adsorption, photolysis and oxidation with hydrogen peroxide or ozone, can generate a large volume of sludge and usually require the addition of other environmental hazardous chemical additives.31–33 Biological treatment technologies are attractive alternatives to the traditional physicochemical methods, as they are low-cost, environmental friendly and can selective to provide a complete degradation of organic pollutants without collateral destruction of either the site’s flora or fauna.2,15,34 Therefore, as better alternatives, biological processes are getting more and more attention since it does not produce harmful byproducts as well as large quantity of sludge.35 Fungi are not only used to decolourize/degrade the hazardous dyes (i.e. azo, anthraquinone, heterocyclic, triphenylmethane and polymeric dyes) but also used to have some beneficial products from the wide range of waste materials, by their bio-transformation.36–38 Investigations on the topic have been emphasized the biodegradation processes. In present review I have tried to explore the research endeavors and achievements as well as advancements in mycoremediation processes targeting the application of fungi in degradation/decolourization of textile dyes and other hazardous effluents.
Fungi and dye degradation
Fungi and bacteria, both are the principal degraders of organic matters, but fungi are better known for the purpose due to their superiority in the enzyme production. Traditionally, fungi have been classified as white-, brown-, or soft-rot fungi on the basis of technical decay and descriptions,39 regardless of their taxonomic position. Because the enzyme systems and metabolic pathways involved in the breakdown of carbohydrates and lignins probably are truly distinct in these fungi, rather than just modified in one or a few specific enzyme activities, decay type is of significant taxonomic importance. One important physiological characteristic of decay fungi in culture is the production of extracellular enzymes phenoloxidases and peroxidases. Certain fungi produce brown diffusion zones in agar plates when supplemented with 0.5%(w/v) Gallic or tannic acid, as a result of oxidation of the respective phenolic acid by extra-or intra-cellular phenoloxidases. Bavendamm40 suggested that the presence of phenoloxidases in white-rot fungi is directly correlated with the abilities of these fungi to the decomposition of lignin. Davidson et al.41 was the first to extend this method to 210 species of wood decaying fungi. Of all tested white-rot fungi, 96% were positive on gallic acid agar, tannic acid agar, or on the both media. Kaarik36 tested 173 wood-decaying species on the plates supplemented with 28 substances and found wide variations in the reactions of given strains to specific phenolic compounds. The basidiomycetous fungus Phanerochaete chrysosporium has an unusual degradative capability and termed as “white rot fungus” because of its ability to degrade lignin, a randomly linked phenylpropane-based polymeric component of wood. The fungus possesses great potential for its commercial use in bioremediation of dyes and lignin-cellulosic materials present in textile effluents. The fungus has become a model example for its uses in mycoremediation and for its biotechnological application in biodegradation. Freitage et al.42 screened 170 strains of white-, brown-, and soft-rot decay fungi and non-decaying xylophilophilous fungi for phenoloxidase activity with the polymeric dye Poly-478, to relate dye decolourization to know ligninolytic activity and the presence of phenoloxidase and peroxydases. On the basis of their growth, these fungi were classified into fast, slow and little growing fungi (Table 1).
White-Rot Fungi |
|
Fast growing fungi |
Bjerkandera adusta, Ceriporiopsis rivulosa, |
Slow growing fungi |
Acanthophysium lividocoerulem, Ganoderma oregonense, |
Little growing fungi |
Acanthophysium lividocoeruleum, Armillaria mellea, |
Table 1: Phenoloxidase activity and categorization of Fungi.42
Wilkolazka et al.43 studied the potential of 115 fungal strains for the degradation/decolourization of structurally different azo and anthraquinonic dyes. They reported Abortiporus biennis, Bjerkandera fumosa, Cerrena unicolor, Clitocybula dusenii, Dichomitus albiodofuscus, Diplomitiporus crustulinus, Flammulina velutipes, Gonoderma lucidum, G. applanatum, Heterobasidion annosum, Keuhneromyces mutabilis, Lentinus edodes, Nematoloma frowardii, Panus tigrinus, Perenniporia subacida, Phanerochaete chrysosporium, Phlebia radiata, Pholiota glutinosa, Pleurotus pulmonarius, Pycnoporus coccineus, Stropharia rugosoannulata, Tremetes sanguinea, T. versicolor, and also Agrocybe cylidracea, Geotrichum sp. Gloeophyllum odoratum, Pestalotia sp., Coprinus micaceus, Fomtopsis pinicola, as potential fungal strains to degrade/decolourize, the tested dyes.
Mechanism of dye degradation: Biodegradation has at least three step; a minor change in an organic molecule leaving the main structure still intact, fragmentation of a complex organic molecule in such a way that the fragments could be reassembled to yield the original structure, and complete mineralization i.e. transformation of organic molecules to mineral forms, including carbon dioxide or methane, plus inorganic forms of other elements that might have contained the original structures.2,7 Ferrell et al.44 compared the colour removal efficiency of lyophilized fungal culture with lignin peroxidase, both the lyophilized culture and enzyme decoloured the dye. A well-defined lignin-degrading enzyme system, consisting of lignin peroxidase, Mn (II)-dependent peroxidase (MnP) and glyoxal-oxidase, was found to be involved in the dye degradation. Adsorption of dyes to the microbial cell surface is the primary mechanism of decolourization. Absorption was found to be important mechanism that contributes to the decolourization process, possibility to initial transformation of the dye degradation.45 Microscopic examinations showed that the fungal spores, instead of the fungal hyphae were actually absorbing the dye. A hydrophobic-hydrophillic interaction between the fungus and the dye might contribute to the absorption phenomenon. The dye colour decreased, when either the enzyme or the cell mass concentrations were increased indicating that the dye degradation occurred both by enzymatic action as well as adsorption to the cell mass. The concerted action of both led to greater degradation than single.
White-rot fungi are unique among eukaryotes in their ability to cleave carbon-carbon bonds in polycyclic aromatic hydrocarbons (PAHs), for the biological degradation of these compounds. The breakdown of most organo-pollutants by ligninolytic fungi is closely linked with ligninolytic metabolism and generally thought that lignin degrading enzymes (whose normal function is lignin degradation) also catalyse the highly nonspecific xenobiotics oxidation that is the characteristic of these fungi. The lignin-degrading system of white-rot fungi includes a family of lignin peroxidases, or ligninases as they are commonly known for the initial oxidative depolymerization of lignin.17,46 Spadaro et al.23 reported that fungus P. chrysosporium is capable to mineralize a variety of toxic dyes and other hazardous aromatic rings compounds, due to the liginolytic activities of the fungi. Several mechanisms have recently been elucidated for the fungi to degrade the synthetic dyes and other organo-pollutants.43,47 The first step in the degradation of many dyes by white-rot fungi often involves the formation of highly reactive free radical intermediates. These free radicals are formed any time; one electron is removed or added to the ground state of a chemical. Such free radicals are very reactive and rapidly give up or abstract an electron from another chemical. This free radical reaction often occurs as chains in which many different radicals are generated subsequent to the formation of the initial radical species. The free radical process provides some basis for the non specific nature of white-rot fungi. The free radical reactions catalyzed by the peroxidases from white-rot fungi also appear to be involved in the degradation of a variety of pollutants.26,45 Paszczynski et al.19 reported the involvement of veratryl alcohol during the degradation of azo compounds. Veratryl alcohol stimulated azo dye oxidation by ligninase, acting as a third substrate (with H2O2 and the azo dye) in the cycle back the enzyme to its native state.
The dye degradation or dye removal process mediated by fungi may be categorized into biosorption, bioaccumulation and biodegradation (Figure 1A‒1D). Adsorption of the dyes to the microbial cell surface is the primary mechanism of decolourization.35,45,48 Wong et al.49 reported the adsorption of Acid green 27, Acid violet 7 and Indigo carmine dyes on living and dead mycelia of Trametes versicolor. Biosorption is a metabolically independent process which involves the binding of solutes to the fungal biomass and thus dye decolourization can occur by either living or dead fungal biomass.50 The dead fungal biomass contains a natural polysachharide chitin and its derivative chitosan in their cell walls which has a unique molecular structure with a high affinity for many classes of dyes. Hence, fungal biomass can be used as efficient absorbent for synthetic dyes. Bioaccumulation is an energy and metabolically dependant process, where actively growing cells accumulate the pollutants inside their cytoplasm (Figure 1A‒1D). Biodegradation is also an energy intensive and metabolically dependant process, where the complex dye molecules are broken down into simpler molecules through the action of certain enzymes. Decolourization of dye is related to the presence of extracellular peroxidases, particularly manganese peroxidases.27
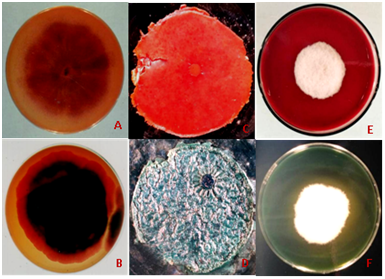
Although dye molecules display high structural diversity, they are only degraded by a few enzymes that share common mechanistic features as they all catalyze redox reactions and, exhibit relatively wide substrate specificities. The most important dye degrading enzymes are: azoreductases, laccases and peroxidases. Azoreductases are oxidoreductases, which are particularly effective in the degradation of azo dyes through reduction of the azo linkage, the chromophoric group of azo dyes.51 These enzymes have a great potential in various biotechnological processes mainly due to their high non-specific oxidation capacity, the lack of requirement for cofactors, and the use of the readily available molecular oxygen as an electron acceptor. These include the detoxification of industrial effluents, mostly from the paper and pulp, textile and petrochemical industries, and bioremediation to clean up herbicides, pesticides and certain explosives in soil.15,52–54 Fungal biomasses walls are composed of macromolecules (chitin, chitosan, glucan, lipid, phospholipides), which contain carboxyl groups (RCOOH), amino groups (R2NH, R-NH2), phosphates, lipids, melanin, sulphates (R-OSO3-) and hydroxides (OH-).55–57 These functional groups are metals sorption sites that play an active role in dye and organo-pollutants degradation.58–60 Fungi are also used for metal removal generally through the adsorption, chemisorptions (ion exchange), complexation, coordination, chelation, physical adsorption and micro-precipitation.55 Metals removal by fungi from various raw effluents (gold mining effluent, tanning effluent, swine water, polluted lake waters) are sometimes completely or partially, depend on the metal and potentiality of fungus involved. When metals are removed by ionic exchange, they generally replace K+, Mg2+, Ca2+ and H+ contained in biomasses.61,62 To increase the removal capacities of fungi some physicochemical treatments such as soda or acidic treatments, insertion of functional groupings, heat treatment are essential.63,64 Kapoor et al.57 also demonstrated that the soda treated biomass of A. niger, removed Cd2+, Cu2+ and Pb2+ with a higher rate of absorption. Yong et al.65 suggested that the binding of dyes to the fungal hyphae, physical adsorption and enzymatic degradation by extracellular and intracellular enzymes, is a major mechanism for the colour removal by the fungi.
Fungal enzymes and their role in dye degradation: Bumpus et al.26 and Barr et al.46 reported that Lentinus edodes (Shiitake), a most popular edible mushroom, produces high amounts of manganes peroxidase (MnP, EC 1.11.1.14). The extracellular production of ligninolytic enzymes by L. Edodes mycelium growing on a solid medium decolorized several dyes such as Poly-478 and Remazol Brilliant Blue R (RBBR). Enzymes such as lignin peroxidase (LiP), manganese-dependent peroxidase (MnP) and laccase, all of which are involved in lignin degradation, have been reported to decolourize the synthetic dyes.28 Kim et al.66 demonstrated the presence of H2O2 dependent enzyme activity to declourize the dye Brilliant Blue R by the fungus Pleurotus ostreatus. Buswell et al.67 reported that the production of the extracellular ligninolytic enzymes is strongly affected by the nature and amount of the nutrients, especially nitrogen (N) and microelements, in the growth substrate. The basidiomycetous fungus Ganodermaa lucidum has been found positive for the removal of Rhodamine-B and Sandolan rhodine dyes. Kim et al.29 purified the novel dye degrading peroxidase (Dyp) from the fungus Geotrichum candidum Dec 1 that was found responsible for the dye- decolourization, of 21 types of dyes; particular anthroquinone dyes. Swamy et al.30 reported the presence of laccase and manganese peroxidase (MnP), during the decolourization of the azo dye (Amaranth), by the fungus Trametes versicolor. The higher percentages of dyes degradation with the presence of laccase produced by T. versicolor and P. ostreatus were also detected by some workers.20,68,69 The identification of two hydroxylated metabolites from the degradation of the most degraded dye, allowed the proposition of a metabolic pathway. White-rot fungi are well known for the production of enzymes for dye degradation as commercial level.33 White-rot fungus, Thelephora sp. was characterized for ligninolytic activity for the dye decolourization/degradation. Bio-degradation/decolourization of several textile dyes including Congo red, Bromophenol blue, Acid red, Direct green by the fungi Aspergillus flavus and Trichoderma harzianum has been reported.6,7,16
Future Perspectives: Based on the investigations, it is clear that within the microbial diversity, fungi are much important for bio-degradation process of hazardous dyes and effluents. Further, investigations on mycoremediation have also been shown an inhibition in decolourizing fungal strain compared to their growth in a normal medium (Figure 1E & 1F) that might be a reason to get a lower percentage of dye degradation/decolourization after using of potential fungal strains for the same. Inhibition in fungal growth during the dye degradation/decolourization against some specific dye compounds clearly indicates the inefficiency of used fungal strain, might be due to toxicity of dye that suppresses the production of enzymes as well as mycelial growth. Applications of molecular tools and technology to identify genes encoding enzymes in potential fungal strains may be helpful in synthetic dye degradation.48 With the help of genetic engineering, genetically modified strains the efficiency of dye degradation/decolurization through fungi may be enhance. Connection among the researchers and policy makers should be come onto the same forum to find out a cheap and environmentally safe large scale treatment process for the industrial effluents. Promotion and set up of interdisciplinary research including chemistry, biochemical and environmental engineering along with microbiology may have success for a permanent biological dye effluent treatment process on a commercial scale, to save and sustain the natural environment from toxic wastes. Proper degradation/decolourization of coloured wastewater from textile industries is major environmental concern. Amongst different chemical, physical and biological treatment methods, the biotechnological approaches based on fungi, are the most effective and environmental friendly methods. Optimization of the culture media in carbon sources or nutrients and mediators molecules is very important to obtain a good output of pollutants degradations.
Scientific developments are considered as key factors for the progress of both developed and developing countries, but unfortunately, most of the industries do not have proper waste treatment facilities or arrangement, and releasing a large quantity of effluents. A definitive solution for colourants problem of textile effluents would provide a marked advantage for the industrial sector. The success of a microbial process for colour removal from the effluent depends on the utilization of microorganisms that effectively decolourize synthetic dyes of different chemical structures. The fungal degradation/decolourization of textile dyes has been demonstrated mainly in the laboratory studies. The fungal utilization in dye decolourization is still under investigation to assess the information’s on process implementation. The results obtained mainly from the laboratory tests depend on specific growth medium and optimization (addition of co-substrate, nutrients, mediator molecules, physical parameters optimization) and a good handling of fungal strains or biomasses. Therefore, essential works on the topic are still in the laboratories or less at commercial scale to solve the problem of colourants in effluents through mycoremediation. The degradation/decolourization and mineralization of recalcitrant dyes and organo-chlorinated compounds are effective by certain fungi. With regard to fungi, which contribute to effluents degradation either by dead mycelia or with enzymes, there is need to design them for the purpose. Commonly dyes removal treatments do not adequately eliminate the azo dyes from the effluent waters of textile mills and dyestuff industries. In order to develop suitable technology to degrade/decolourize synthetic dyes discharged in the effluent and to convert them into beneficial products simultaneously, a well-planned scientifically acceptable technology is needed. The waste fungal biomass from fermentation industry is also a good absorbent that used as bio-sorption for biological effluent treatment in dyes and textile sectors. Enzymatic processes are particularly sought for the treatment of dye-containing effluents, mainly because of their specificity and relatively ease of engineering towards improved robustness; enzymes only “attack” the dye molecules, while valuable dyeing additives or fibers are kept intact and can potentially be re-used. Likewise, new recycling technologies will allow a huge reduction in water consumption in the textile finishing industry. One of the most important factors, which have a great impact on the setting of a proper bioremediation plant for textile wastewater, is the effluent characteristics. Majority of the researches concerning the fungi for mycoremediation of textile wastewaters have been focused the potentiality of fungi in dyes degradation/decolourization while the limitation of these fungi against dye compounds yet haven’t been examined properly.
Author is thankful to Head, Department of Botany, University of Delhi, Delhi providing necessary facilities during the laboratory work. I am also thankful to Mr. Harsh Kumar Chauhan and Hardesh Kumar, DSB Campus, Kumuan University, Nainital for their help during the manuscript preparation.
The author declares no conflict of interest.

©2017 Singh. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.