Journal of
eISSN: 2572-8466


Research Article Volume 1 Issue 1
1Department of Chemistry & Biochemistry, University of Windsor, Canada
2Holland Marsh Grower's Association (HMGA), 50 Barrie Street, Canada
3Upper Thames River Conservation Authority (UTRCA), Canada
4Ontario Ministry of Agriculture, Food & Rural Affairs (OMAFRA), Canada
Correspondence: Bulent Mutus, Department of Chemistry & Biochemistry, University of Windsor, 401 Sunset Ave, Canada, Tel 519-253 3000, Fax 3526
Received: September 04, 2016 | Published: October 5, 2016
Citation: Yep T, Saunders M, Merkley C, et al. Application of red-sand/chitosan hybrid filtration system for phosphate removal from agricultural wastewater. J Appl Biotechnol Bioeng. 2016;1(1):28-33. DOI: 10.15406/jabb.2016.01.00005
Point sources of phosphate used in this study were from a livestock transport truckwash station, and a vegetable wash operation. Two types of red sand/chitosan hybrid filtration systems were used:
Keywords: chitosan, red sand, filtration, wastewater, phosphates, ion exchange
The accumulation of phosphates and nitrates in wastewater derived from various sources including, agricultural processes and livestock farming, is eventually discharged into oceans, lakes and rivers. A most common reason for this is usually due to a lack of infrastructure or cost aversion. This leads to algal blooms, and eventually to eutrophication, adversely affecting water quality and eco-systems.
In recent years, several techniques have been employed for the treatment of phosphates which includes chemical precipitation, biological treatment, as well as ion exchange.1-7 The currently employed techniques are very effective at attenuating phosphates from wastewater effluents however, they require a significant investment of capital and space.8,9 Constructing wetlands10 and reed beds11 were some alternative techniques employed for the treatment of phosphates which are relatively cheaper, when it is compared to conventional strategies. Ion exchange is an alternative option for wastewater treatment due its capacity to sorb ions of interest which can be desorbed at a later stage for applications in other aspects such as manure production.12 Common ion exchangers include zeolites13 and synthetic polymers14 that contain modified functional groups to remove positively or negatively charged ions depending on the nature of the functional group. Chitosan, a biopolymer obtained by the deacetylation of chitin. It has applications in ion exchange due to its ability to selectively sorb transition metal ions.15 Chitosan is structurally similar to cellulose except that it has primary amines present at the C-2 position.16 This hetero-polymer’s amines and hydroxyl groups present on the polymer participate in the formation of dative bonds with transition metal ions.16-19 Chitosan had mostly been used as a coagulant in wastewater treatment applications however, metal-complexed chitosan has been shown to act as an ion exchange medium for anionic contaminants like phosphates, and research on its application in wastewater treatment is currently underway.20-22 Sand based filtration systems have been used for the tertiary treatment of water23 and are usually used to remove organic matter and suspended solids from influent wastewater streams. This type filtration system has also been used in conjunction with UV irradiation in order to treat wastewater effluents to produce potable water.24 Red sand is a mixture of sand and red sandstone which is rich in hydrated iron oxide which is responsible for its red color. It is commonly found in areas containing red sandstone.
The present study is aimed at studying the effectiveness of a hybrid sand/chitosan filtration system for treating wastewater from agricultural sources that is focused on attenuating soluble phosphate content as well as suspended solids in the filtrate.
Ascorbic acid (Sigma), Ammonium molybdate tetrahydrate (Sigma), Chitosan flakes (Dungeness environmental), Dipotassium phosphate (Fisher Scientific), Iron-complexed chitosan flakes (Chemfil Canada Ltd), Malachite Green Carbinol hydrochloride (Sigma), Iron (II) sulfate (Sigma), Polyvinyl alcohol (Sigma), Red sand (Hutcheson Sand & Gravel), Sulfuric acid (Sigma), Whatman filter paper (Fisher).
Preparation of iron-complexed chitosan flakes
Chitosan (DOD% 85) was mixed with 0.1M iron sulfate solution. The flakes were mixed at 350rpm in the iron sulfate solution incubated for 3 hours after which, the flakes, were washed with distilled H2O, three times,10X chitosan volume, were dried in an oven at 50°C for 3 hours.
Study sites and experimental design
Filtration apparatus was tested at a livestock transport truckwash station located in Sebringville, Ontario and a vegetable wash station located in Ansnorveldt, Ontario. Locations were chosen by local conservation authorities based on the urgency for phosphate treatment as both sites exhibited a high concentration of phosphate and suspended solids content (Figure 1).

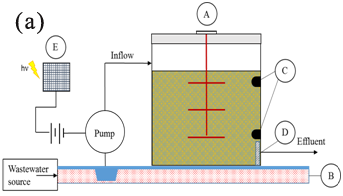
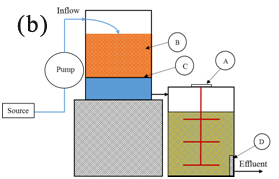
Sampling
Wastewater samples were dependent on the wash cycles at both locations. The “initial” readings were taken during the beginning of every wash cycle. Wastewater was grab sampled from the artificial wetlands constructed at both sites as well as from the post treatment effluent at 8 hour intervals over 3 weeks. The samples (50 mL) were then acidified with sulfuric acid and stored in polyethylene bottles, which were transported in iced cooler boxes. Samples were analyzed within 24 hours of collection.
Chemical methods
Phosphate desorption studies for iron-complexed chitosan and red sand
Saturating chitosan flakes with metals significantly improved its phosphate sorption capacity. In these field tests, chitosan was saturated with iron and its phosphate binding capacity based on desorption studies are shown in Table 1. In addition to this, it was observed (Table 1) that red sand was not a very effective adsorbent for phosphates. The adsorption of phosphates could be due to the presence of aluminosilicates in the mixture, which are usually the main components of zeolite matrices.
Filter |
Phosphate Recovered (mM) |
Phosphates recovered (mg /g Chitosan) |
Native chitosan flakes |
5.59 ± 0.57 |
10.84 ± 1.07 |
Iron-complexed chitosan flakes |
8.79 ± 0.23 |
17.05 ± 0.44 |
Red sand |
0.11 ± 0.01 |
0.28 ± 0.02 |
Table 1 Comparison of chitosan flakes with iron chitosan flakes and ‘red sand’ indicates the adsorption capacity of iron-complexed chitosan is higher than the other filters being used for water treatment. All experiments were performed in triplicates and values are the average concentration of phosphate from 3 separate experiments. The error bars represent standard error (n=3).
Truckwash station
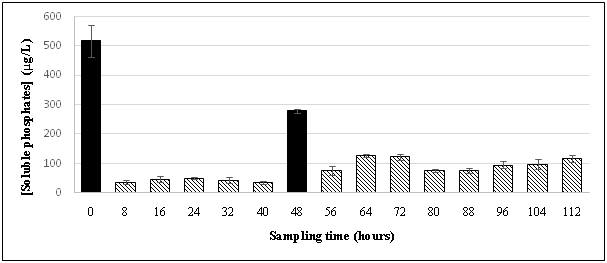
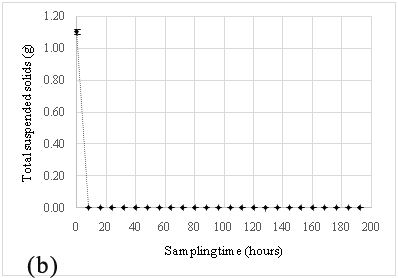
The hybrid red-sand/chitosan filtration system was first applied at this site, and during the sampling periods May-July 2015, the filtration system had demonstrated its effectiveness in attenuating a significant amount of phosphates from the truck wash effluents shown in Figure 2A and 2B. The pre-filter, red sand effectively removed ~99% of TSS from wastewater in the effluent as shown in Figure 5 and along with reducing the soluble phosphates from the settling pond by about 6–30 fold (~500 µg/L) prior to the introduction into the chitosan filtration unit. This suggested that the red sand pre-filter was either adsorbing or precipitating all the soluble phosphates after the effluent had passed through it. Subsequently, the chitosan filtration unit was able to lower it further to ~200 µg/L.
Vegetable wash station
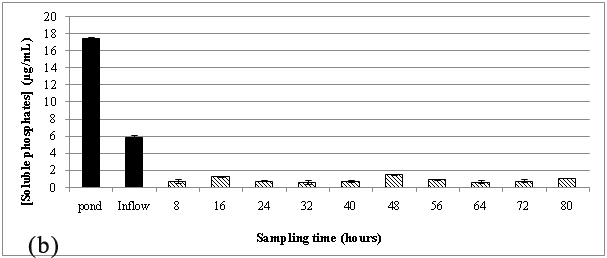
The hybrid filtration system was used to treat a similar type of wastewater at this site. This site introduced the sloughs of sediment and organic matter from the vegetable wash effluents after every vegetable wash into the waste streams. A similar trend was noticed when compared to the results observed at the truck wash station during the sampling periods of October to November 2014 Figure 3 and April to May 2015 Figure 4, where the wastewater source was high in phosphate content and after it allowed to flow through red sand, sediment-free wastewater was subsequently introduced into the chitosan filtration unit where there was a further reduction in the phosphate concentration. The gravity filtration of the wash effluents was able to reduce phosphate in wash effluents from ~14 μg/mL to ~1 to 2 μg/mL after passing through red sand as shown in Figure 3A & Figure 4A. The chitosan filter unit connected in tandem, further lowered phosphate levels to ~ 0.5 μg/mL.
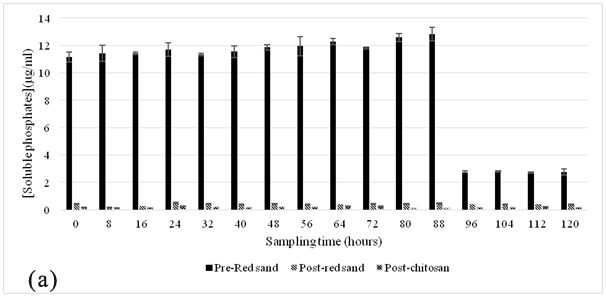
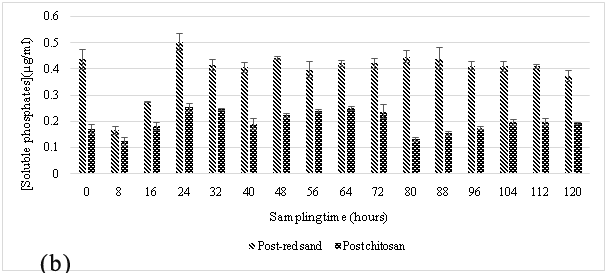
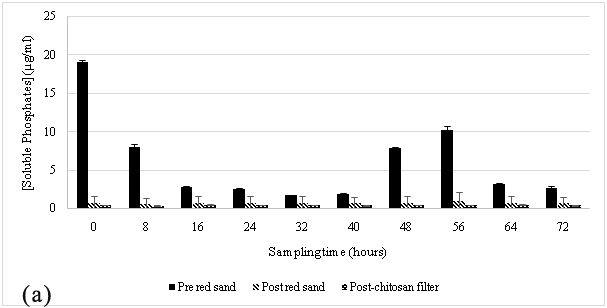
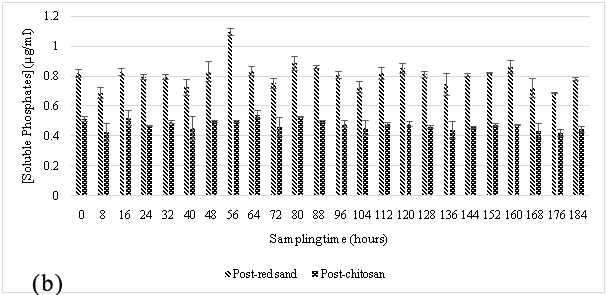
At both sites, it was observed that the red sand pre-filter had removed almost all suspended solid content and the result was reflected in the sharp decrease in soluble phosphates in the effluent samples. The particulate size of the filter made it ideal for the separation of suspended solids from the influent wastewater stream. This sharp decrease in phosphate concentration was most likely due to the filtration of soil-bound phosphates by red sand as the effluent stream passed through it.25,26 In addition to this, an attempt to regenerate the spent red sand, regeneration studies were performed with 1M ammonium acetate and 1M sodium chloride. These results, as shown in Table 2 suggest that red sand had also contributed to the adsorption of soluble phosphates from the influent wastewater. However, the amount adsorbed suggests it cannot be used for ion exchange applications for phosphate contaminated wastewater. A similar setup has been used in other studies where sand was used for a similar purpose and was very effective in removing phosphorous10 however, the additional chitosan filtration unit connected in sequence with the red-sand filter makes it possible to adsorb soluble phosphates in the effluents. The result would ensure that the water quality meets the standards for the region (PWQO Ontario).
Regenerant |
Phosphates Recovered (µg/ml) |
NaCl |
4.51 ± 0.03 |
CH3COONH4 |
3.56 ± 0.02 |
Table 2 Red sand (55 kg) was treated with regeneration solution (500 ml) (1M ammonium acetate or 1M sodium chloride). All values represented here were average phosphate concentration of 3 separate sampling events. The error bars represent standard error (n=3).
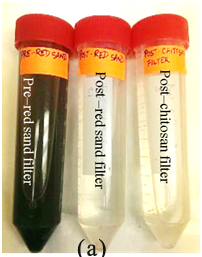
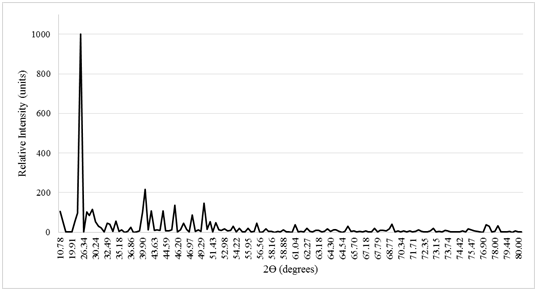
Chemical composition of red sand (pXRD data)
The composition of the sand would give an insight on the processes that make red sand such a potent phosphate adsorbent. Upon analysis by XRD, the sand being used was comprised of various minerals and the peaks of interest matched closely with lithium aluminosilicate with an FOM of 0.83,27 and other minerals such as CsCu2I328 and Tin (II) pyrophosphate.29 This investigation suggested that adsorption played a small role in the removal of phosphates. In addition to this, the mixture did not contain any iron salts and so, it must be removing phosphates using a different process when compared to iron-chitosan flakes (Figure 6).
Regenerative studies of red sand
The results from the treatment of red sand with regenerant solution suggested that red sand adsorbed a small amount of soluble phosphates when compared to iron-chitosan flakes. The average soluble phosphates recovered from in the spent regeneration solution are shown in Table 2.
The hybrid red-sand/chitosan filtration system is a cost effective method to treat agricultural wastewater containing high TSS and phosphate content. The TSS content can be removed from the pre-filter unit and be reused for agricultural purposes as it will still contain high concentration of soil bound phosphates. Spent chitosan can be treated with ammonium acetate to elute the bound phosphates from the column and by precipitating it as struvite, it has a potential application in fertilizer production.
None.
The author declares no conflict of interest.

©2016 Yep, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.