Journal of
eISSN: 2572-8466


Background: Cyclosporine A (CsA) is a compound widely used as an immunosuppressive drug, particularly, in case of heart, liver and kidney transplantation to prevent rejection of transplanted organ. Toxic effects of CsA have been demonstrated in a variety of organs of experimental animals, including kidney, liver, hematopoietic systems, the lymphoid system, the alimentary tract, and skin.
Objectives: This study aimed to investigate the side effect of acute and chronic treatment with CsA on haematological parameters and serum proteins in male albino rats.
Materials and Methods: Eighty male albino rats were divided into 4 main groups, each one divided into two subgroups, consisted of 10 animals. The first group were divided into 2 subgroups, the first serves as a control group, and the second were treated orally with 20 mg/Kg/day CsA for one week. The second group were divided into 2 subgroups, the first serves as a control group, and the second were treated orally with 20 mg/Kg/day CsA for two weeks. The third group were divided into 2 subgroups, the first serves as a control group, and the second were treated orally with 20 mg/Kg/day CsA for four weeks. The fourth group were divided into 2 subgroups, the first serves as a control group, and the second were treated with one acute dose (50 mg/Kg) of CsA orally. Blood samples were obtained for assessment of haematological parameters and serum proteins.
Results: The results of the present study showed that, chronic treatment with CsA for 4 weeks caused a highly significant (P<0.01) decreases in RBCs count, hemoglobin concentration, haematocrite value, MCHC, Lymphocytes%, and increase in MCV, MCH, and neutrophils % as compared to control. Acute administration of CsA caused a significant decreases (P<0.05) in eosinophils % and (P<0.01) basophils when compared to controls. Also, chronic administration of CsA for four weeks caused a highly significant (P<0.01) decreases in serum albumin, A/G ratio, and increase in serum Globulins as compared to control groups.
Conclusion: It can be concluded that, chronic cyclosporine A had adverse effects on haematological parameters, serum total proteins, albumin, globulin, and A/G ratio in male albino rats.
Keywords: cyclosporin A, haematological parameters, RBCs count, WBCs count, leucocytes differential count, serum proteins, rats
Cyclosporine A (CsA) is isolated from a fungus Tolypocladium inflatum gams. It has been exerted a biologic activities, including anti-inflammatory, a fungicidal, and anti-parasitic effects, and immunosuppressive agent.1,2 It is a new generation of immunosuppressive drugs (calcineurine inhibitors) that inhibit the lymphocytes specifically, and reversibly.3,4 Calcineurine inhibitor is used to prevent rejection episodes in patients after transplantation of heart,4–6 liver,7 and kidney.8 Therefore, CsA is used to treat autoimmune diseases like psoriasis, nephritic syndrome, rheumatoid arthritis, and atopic dermatitis and prevent the rejection of organs transplantation.9,10 After binding of CsA with cyclophilin in cytoplasm, it interferes with a calcineurin (Calcium calmodulin dependent serine/threonine protein phosphatase). Therefore, calcineurin cannot dephosphorylate the nuclear factor of activated T cells in the cytoplasm and leads to decrease IL-2 gene transcription in the activated T cells.8,11,12 The nuclear factor of activated T cells activates the transcription of cytokines which promotes the proliferation and growth of B and T -cells. T lymphocytes produced IL2 in response to mitogenic or antigenic stimulation which is also necessary for the proliferation and differentiation of many immune cells including natural killer cells, B lymphocytes, activated T lymphocytes, lymphokine-activated killer cells, and macrophages.8,13 CsA inhibits the production of interleukins 1a &1b, interleukin 6, γ-interferon, and other lymphokines,8,14 which stimulate the hematopoiesis, modulate the inflammatory and immune reactions, and regulate immunity.8,15
Toxic effects of CsA have been demonstrated in a variety of organs of experimental animals, including kidney, liver,8,16 hematopoietic systems, lymphoid system, alimentary tract, and skin.16 It has been reported that, plasma cyclosporine concentration was correlated with drug toxicity.17 Treatment of patients with immunosuppressive drugs may be leads to haematological toxicity,18,19 nephrotoxicity4 which is a common problem in thirty percent of patients treated with cyclosporine A.2
This study aimed to investigate the side effect of treatment with cyclosporine A on haematological parameters and serum proteins in male albino rats.
Experimental animals
80 male albino rats weighting 100–120 g were used as experimental animals in the present study. The rats were housed individually in plastic cages and acclimated for 7 days. Food and water were offered ad libitum. Animals were maintained at 22± 2°C at normal light/dark cycle.
Experimental design
Cyclosporine A drug was purchased as Sand immune capsules (Novartis International AG, Basel, Switzerland). It was emulsified in olive oil and administered orally a single dose (50mg/Kg) in acute case and 20mg/Kg/day in case of chronic dosages.
The rats were randomly selected and divided into four main groups, each divided into two subgroups, consisted of ten rats as follow:
Blood collection
24 hours after stopping treatment with cyclosporine A, animals were anaesthetized by diethyl ether, dissected and blood was collected by heart puncture with syringe (3ml capacity). The required amount of blood was collected in tubes, and the blood allowed to coagulate in water bath at 37°C for 30 minutes. Serum was separated by centrifugation in cooling centrifuge (Hettich, Germany) at 3000xg for 15 minutes, transported into another dry and clean Eppendorf tubes and was kept in deep freezer at –20°C for biochemical analysis.
Haematological analysis
Hemoglobin in blood was determined according to method of Van Kmpen & Zijlstra20 using the kit of Randox Company, United Kingdom. Red blood cells and white blood cells were counted using hematocytometer method according to Krupp et al.,21 PCV percentage was determined according to Turgeon22 using microhematocrit tubes coated with anticoagulant. And Leukocyte differential counted was preceded according to Turgeon22 by using Giemsa stain (Atlas Medical Company, UK).
Biochemical analysis
Serum total protein and albumin analysis were performed using commercially available kits from Diamond Diagnostics Company (Egypt) according to the manufacturer’s instructions.
Statistical analysis
Data were expressed as mean±SD. The level of statistical significance was taken at P <0.05, using one way analysis of variance (ANOVA) test followed by Dunnett test to detect the significance of differences between each group and control. All analysis and graphics were performed by using, INSTAT and graph Pad Prism software version 6.
Data in Table 1 & Figures 1–6 shows RBCs count, Hb, Hct, MCV, and MCHC in rats treated with 20 mg/kg/ day CsA for 1, 2 & 4 weeks, and controls. Treatment of rats with CsA for 4 weeks caused a significant decreases (p<0.01) in RBCs count, Hb, Hct, and MCHC, -39.5%, -20.6%, -10.5% & -11.3%, respectively, but MCHC was a significantly decreased (p<0.05) after 2 weeks of treatment (-3.9%) when compared with controls (Figures 1–3). On the other hand (Figure 6), MCV was a significantly increased (p<0.01), 5.2 %, and 48.2%, respectively after 2, and 4 weeks of treatment, but MCH was significantly increased (p<0.01) (31.3%) after 4 weeks only in rats treated with CsA as compared with controls (Figures 4&5). WBCs count was a significantly decreased (p<0.05) (-12.7) in rats treated for 4 weeks compared with controls (Figure 7). Rats treated with CsA for 2 & 4 weeks showed a significant decrease (p<0.05) in lymphocytes% (-2.9%), and (p<0.01) (-26%), respectively as compared with controls (Table 2) (Figure 8).
Treatment Periods |
One week |
Two weeks |
Four weeks |
Acute dose |
||||
Control Mean±SD |
Treated Mean±SD |
Control Mean±SD |
Treated Mean±SD |
Control Mean±SD |
Treated Mean±SD |
Control Mean±SD |
Treated Mean±SD |
|
RBCs (106 xCells/µl) |
4.02±0.30 |
4.00±0.36 |
5.33±0.33 |
5.00±0.36 |
6.93±0.25 |
4.19**±0.30 |
7.45±0.38 |
7.37±0.35 |
Hb (gm/dl) |
11.92±0.57 |
11.46±0.5 |
11.60±0.5 |
11.00±0.43 |
12.46±0.83 |
9.90**±0.98 |
13.36±0.49 |
13.34±0.49 |
Hematocrit % |
37.00±2.97 |
36.66±2.53 |
37.00±2.00 |
36.50±2.12 |
39.66±0.88 |
35.50**±1.40 |
42.60±2.81 |
41.90±1.47 |
MCV (μ3) |
92.02±0.41 |
91.74±1.53 |
69.44±0.43 |
73.04**±0.81 |
57.24±0.63 |
84.82**±2.17 |
57.16±0.68 |
56.87±0.63 |
MCH (pg) |
29.68±0.63 |
28.71±1.06 |
21.78±0.32 |
22.03±0.58 |
17.97±0.43 |
23.60**±0.51 |
17.86±0.20 |
18.11±0.15 |
MCHC (g/dl) |
32.26±0.83 |
31.29±0.63 |
31.36±0.27 |
30.15*±0.45 |
31.40±1.10 |
27.85**±1.32 |
31.25±0.72 |
31.84±0.08 |
Table 1 Effects of chronic or acute treatment of albino rats with cyclosporine A on RBCs count and its indices
*, significant difference compared to control group (P < 0.05); **, highly significant difference compared to control group (P < 0.01).
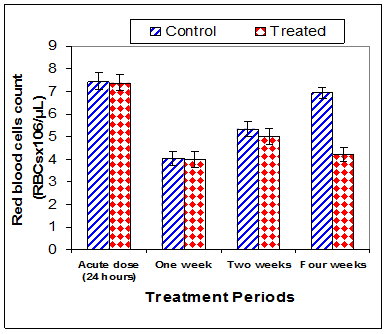
Figure 1 Effect of acute or chronic treatment of albino rats with cyclosporine A on red blood cells count.
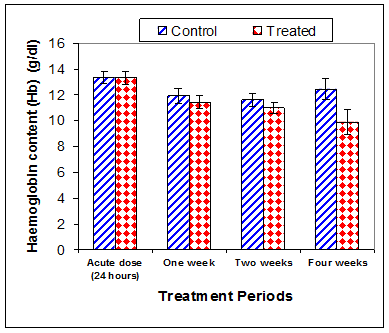
Figure 2 Effect of acute or chronic treatment of albino rats with cyclosporine A on haemoglobin content.
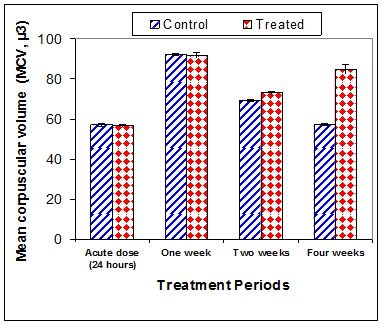
Figure 4 Effect of acute or chronic treatment of albino rats with cyclosporine A on mean corpuscular volume.
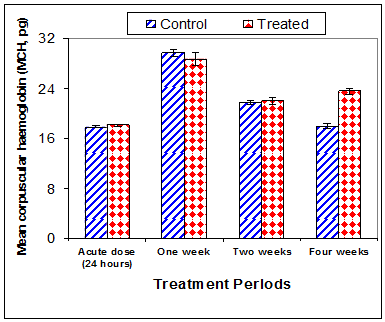
Figure 5 Effect of acute or chronic treatment of albino rats with cyclosporine A on mean corpuscular haemoglobin.
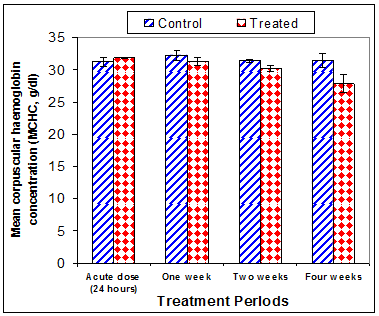
Figure 6 Effect of acute or chronic treatment of albino rats with cyclosporine A on mean corpuscular haemoglobin Concentration.
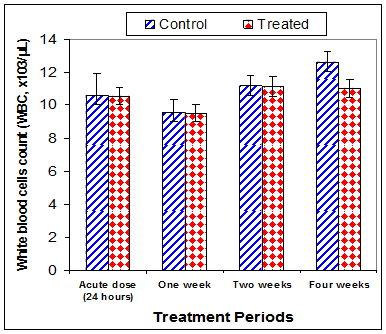
Figure 7 Effect of acute or chronic treatment of albino rats with cyclosporine A on white blood cells count.
Treatment Periods |
One week |
Two weeks |
Four weeks |
Acute dose |
||||
Control Mean±SD |
Treated Mean±SD |
Control Mean±SD |
Treated Mean±SD |
Control Mean±SD |
Treated Mean±SD |
Control Mean±SD |
Treated Mean±SD |
|
WBCs (103 xCells/µl) |
9.58±0.80 |
9.53±0.53 |
11.20±0.61 |
11.14±0.59 |
12.63±0.55 |
11.03*±0.60 |
10.58±1.35 |
10.56±0.53 |
Lymphocytes% |
73.2±2.5 |
68±2.1 |
74.6±1.9 |
65*±2.3 |
77±2.3 |
57**±2 |
74±1.85 |
75±2.5 |
Neutrophils % |
20.6±1.82 |
24.8*±2.1 |
19.6±2 |
28.2**±1.95 |
18.2±1.9 |
38**±2.2 |
20.1±1.85 |
20±1.9 |
Monocytes % |
4.3±0.11 |
5.1*±0.15 |
4.3±0.15 |
4.7*±0.1 |
3.5±0.17 |
3.8±0.12 |
3.8±0.14 |
3.7±0.17 |
Eosinophils % |
1.3±0.15 |
1.4±0.1 |
1±0.1 |
1.4*±0.18 |
0.9±0.08 |
0.8±0.1 |
1.4±0.15 |
0.9*±0.25 |
Basophils % |
0.6±0.05 |
0.7±0.08 |
0.5±0.07 |
0.7*±0.09 |
0.4±0.08 |
0.4±0.07 |
0.7±0.09 |
0.4**±0.08 |
Table 2 Effects of chronic or acute treatment of albino rats with cyclosporine A on leucocytes count and differential count
*, significant difference compared to control group (P < 0.05); **, highly significant difference compared to control group (P < 0.01).
Chronic treatment of rats with CsA induced a significant increase (p<0.05) in neutrophils % after one week (20.4%) and (p<0.01) after 2 & 4 weeks, 43.9% and 102.8%, respectively as compared with controls (Figure 9). Also, monocytes % was a significantly increase (p<0.05) in rats treated with CsA for 1 & 2 weeks 18.6% & 9.3%, respectively when compared with controls (Figure 10). Treatment of rats with a single dose of CsA induced a significant decrease (p<0.05) in eosinophils % and (p<0.01) basophils % , -35.7% & -42.9%, respectively when compared with controls, but conversely treatment of rats with CsA for 2 weeks caused a significant increase (p<0.05) in eosinophils % ( 40%)& basophils % ( 40%) as compared with controls (Figures 11 & 12).
Total protein concentration was significantly decreased (p<0.01) two weeks post CsA treatment (-11.2%) compared to control (Table 3) (Figure 13). Also, serum albumin was a significantly decreased (p<0.01) (-16.6% ) after two weeks and (-26.23%) four weeks of treatment (Figure 14). On the other hand, serum globulin was significantly increased (p<0.01) after 4 weeks of treatment (Figure 15). A/G ratio showed a significant decreased (p<0.01) four weeks post CsA treatment (Figure 16). However, no significant change in serum proteins were recorded in case of acute treatment of rats with CsA as compared with controls (Table 3) (Figures 13–16).
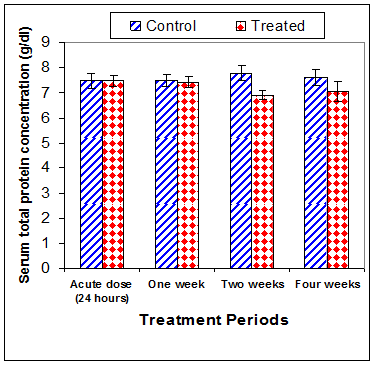
Figure 13 Effect of acute or chronic treatment of albino rats with cyclosporine A on serum total protein concentration.
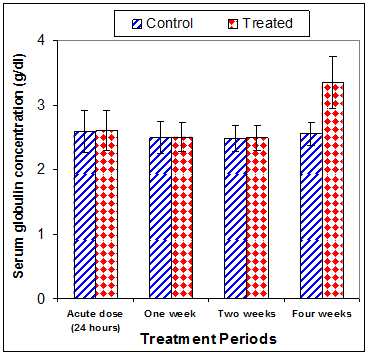
Figure 15 Effect of acute or chronic treatment of albino rats with cyclosporine A on serum globulin concentration.
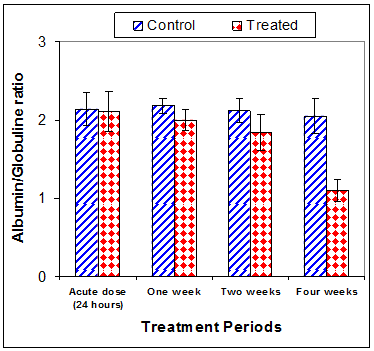
Figure 16 Effect of acute or chronic treatment of albino rats with cyclosporine A on Albumin/ Globulin ratio.
Treatment Periods |
One week |
Two weeks |
Four weeks |
Acute dose |
||||
Control Mean±SD |
Treated Mean±SD |
Control Mean±SD |
Treated Mean±SD |
Control Mean±SD |
Treated Mean±SD |
Control Mean±SD |
Treated Mean±SD |
|
Total protein )gm/dl) |
7.49±0.25 |
7.42±0.21 |
7.78±0.30 |
6.91**±0.19 |
7.60±0.33 |
7.06±0.39 |
7.49±0.29 |
7.48±0.22 |
Albumin (gm/dl) |
5.00±0.15 |
4.90±0.22 |
5.30±0.14 |
4.42**±0.25 |
5.05±0.17 |
3.71**±0.43 |
4.90±0.19 |
4.88±0.33 |
Globulins (gm/dl) |
2.49±0.25 |
2.50±0.22 |
2.48±0.20 |
2.49±0.19 |
2.55±0.18 |
3.35**±0.40 |
2.59±0.33 |
2.60±0.31 |
A/G ratio |
2.18±0.10 |
2.00±0.13 |
2.12±0.15 |
1.84*±0.23 |
2.05±0.22 |
1.10**±0.14 |
2.14±0.21 |
2.11±0.26 |
Table 3 Serum proteins as affected by chronic or acute treatment of albino rats with cyclosporin A
*, Significant difference compared to control group (P < 0.05); **, highly significant difference compared to control group (P < 0.01).
The current study has revealed that treatment of rats with CsA for four weeks caused a significant decreases (p<0.01) in RBCs count, Hb, Hct, and MCHC and MCHC was significantly decreased (p<0.05) after 2 weeks of treatment when compared with controls. These results are in accordance with previous studies. Oral treatment of male Lewis rats with 10–20mg/kg mycophenolate mofetil (an immunosuprisive drug), for two weeks induced a significant reduction of RBCs count.23 There were a significant inverse correlation between the plasma concentration of cyclosporine and hematocrite.17 A clinical reports showed that a significant decreased in Hb concentration following an increase in the plasma concentration of cyclosporine.17,24 The most common cause of unexplained anemia may be due to bone marrow suppression induced by Immunosuppressive drugs.
Elsayed et al.,8 reported that chronic treatment of rats with CsA induced nephrotoxicity by increasing Kidney function parameters. The relationship between anemia and chronic kidney disease is thoroughly documented.26 Also, anemia is common after treatment with cyclosporine in the liver transplanted patients which may be due to inhibition of erythropoietin production induced by cyclosporine.7 Also, erythropoietin production was reduced when cyclosporine added to the culture medium of the human Hep3B cell line.27 In activated T cells, cyclosporine has the ability to block the transcription of cytokine genes, through the inhibition of the phosphatase activity of calcineurin, which subsequent activation of NFAT transcription factor and regulates nuclear translocation. Also, cyclosporine blocks the JNK, NFkB, and p38 signaling pathways.28 It induces the transcription of set of the genes controlling extracellular matrix deposition and gene of TGF beta.29,30 Tacrolimus and cyclosporine have several effects on the growth factors and production of cytokines. Both immunosuppressive drugs are impair the production of IFN gamma, interleukin 2, and interleukin 3.31,32
Cyclosporine exerts its main pharmacological effect as an immunosuppressive by binding to a member of intracellular protein known as immunophilin, forming a complex that interferes with signalling pathway important for the clonal expansion of lymphocytes. It blocks proliferation of T-cell by inhibiting the phosphatase activity of a Ca2+ activated enzyme at nanomolar concentration. The compound binds to the cyclophilins and the cyclophilin-drug complex binds and inhibits the calcineurin.3,33 When intracellular Ca2+levels rise calcineurin dephosphorylates the NFATc in the cytoplasm, which migrate to the nucleus to form complexes with nuclear partners, and induce transcription of genes including those for IL2, CD25, CD40 ligand, Fas ligand and interferon (IFN).3
In the current study, cyclosporine A treatment induced a decreases in WBCs count, and lymphocytes %. On the other hand, chronic treatment of rats with CsA induced a significant increase (p<0.05) in neutrophils % after 1, 2 & 4 weeks as compared with controls. Also, monocytes % was a significantly increase (p<0.05) in rats treated with CsA for 1 & 2 weeks when compared with controls. This changes are in accordance with the previous studies which reported that the immunosuppressive agent, mycophenolate mofetil, caused leucopenia with a lymphopenic effect.18,19,23
Bone marrow is a source of blood cells involved in immune activity because it is the site of proliferation and turnover of blood cells. It is the organ most affected by immunosuppressive drugs, which may be decrease the ability of bone marrow to produce new blood cells leads to leucopoenia.34,35 The mechanisms of haematotoxicity induces by immunosuppressant agents are cross reactivates of antithymocyte globulin/anti-lymphocyte globulin, OKT3, lymphocytolytic effect and inhibition of signal transduction pathways, including mitogenactivated protein kinase, AKR mouse T-cell lymphoma, phosphatidyl inositol 3-kinase, mammalian target of rapamycin/p70S6 kinase, which are involved in the suppression of apoptosis. Also, inhibition of nucleic acid synthesis may leads to bone marrow suppression.19 de novo purine and nucleic acid synthesis are suppressed by MPA, a selective, reversible, noncompetitive antagonist of inosine monophosphate dehydrogenase (IMPDH) activity of bone marrow cells and lymphocytes.19,36 The inhibition of IMPDH activity by MPA may be leads to the inhibition of cell proliferation or induction of apoptosis.37 The results of the current study showed that a significant decrease in serum total proteins and albumin were observed in rats treatmed with CsA for two weeks. The decreases in serum total proteins and albumin may be due to toxicity of CsA on the liver. According to the study of Mostafavi–Pour et al.,38 who reported that CsA induced hepatotoxicity may be due to a decrease in α2β1 integrin expression as a direct effect of CsA or from overproduction. The mechanism of hepatotoxicity of CsA is prevention of the mitochondrial permeability transition pore from opening leading to oxidative stress.39
It can be concluded that, chronic cyclosporine A had adverse effects on haematological parameters and serum total proteins, albumin, globulin, and A/G ratio in male albino. Further studies are necessary to elucidate exact mechanism of haematotoxicity of cyclosporine A.
None.
The author declares there are no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.