Journal of
eISSN: 2572-8466


Cola is a globally distributed and consumed beverage. Soft drinks may contribute significantly to human health conditions and diseases associated with the Western Diet, consisting mainly of a high-volume intake of processed sugars, red meats and high fat foods. Ingredients comprising this beverage are undefined and may have an impact on the behavior of normal human cells, including the controlled cell proliferation, cell cycle and innate expression of ⍺5β1 integrin expression of human foreskin fibroblasts (HFF-1). The proliferation of HFF-1 was examined in the presence of an array of concentrations of Cola supplementation (0.5%, 1.0%, 1.5%, 2.0% 6.0%, 8.0%, and 10.0%) in conditions defined by the initial presence of 10μg/mL fibronectin. In the presence of increasing concentrations of Cola a decrease in the proliferation of HFF-1 cells is observed. Additionally, there are an increased proportion of HFF-1 cells in the G1 phase in an environment consisting of increasing concentrations of Cola. There was no observed increase or decrease in the α5 or β1 integrin expression. With these findings, we suggest that more research should be conducting to better understand the effects resulting from Cola in larger concentrations.
Keywords: collagen, fibronectin, proliferation, morphology, RGD, integrins, cell behavior
HFF, human foreskin fibroblasts; ECM, extracellular matrix; DMEM, Dulbecco’s modified eagle’s medium; FBS, fetal bovine serum; PBS, phosphate buffer saline; FACS, fluorescent activated cell sorting
Sweetened beverage intake by Americans has increased 135% from 1977 to 2001.1 Cola soft drinks are predominantly composed of varying chemicals which function as preservatives, colorings, and artificial flavoring.2 However, due to trade secrets, there are unidentified ingredients included, such as ‘merchandise 7x’. Increased consumption of fructose concentrated beverages is a key component in the classification of the Western Diet, characterized by high intake of saturated and omega-6 fatty acids, reduced omega-3 fat intake, an overuse of salt, and processed sugars.3,4 The overconsumption of these beverages may have links to the risk in numerous human health disorders including obesity, dental/bone disorders, diabetes mellitus, and cardiovascular disease.5 Ingredients comprising these beverages may have an impact on the behavior of key cell types critical in wound healing.6‒12
Fibroblasts are spindle-shaped, anchorage dependent, members of the connective-tissue family. Fibroblasts secrete type I and III collagen into the extracellular matrix (ECM) to help isolate and repair damaged tissue.13 Fibroblasts bind directly to fibronectin fibrils which subsequently attach to collagen scaffolds which provide structure, support, growth and communication.14 The ECM is made of growth factors and proteins that directly affect the differentiation, proliferation, and migration of cells.15 Fibronectin is a ubiquitous ECM glycoprotein, joined by disulfide bonds, that are assembled into a fibrillar matrix. Fibronectin promotes the recruitment of platelets, neutrophils, monocytes, fibroblasts, and endothelial cells to wounds.16 Integrins tether fibronectin dimers initiating fibronectin-fibronectin interactions creating an insoluble fibrillar ECM that aids proliferation, adhesion, and migration of cells during the wound healing process.16‒18
Hypothesis
Due to the ingredients comprising of cola beverages, these intricate mechanisms may become manipulated, allowing for human health issues involved with cell proliferation and migration.7‒9 Imbalance within these processes may result in the delay of tissue regeneration process. Soda cola concentrations, 0.5, 1.0, 1.5, 2.0, 4.0, 6.0, 8.0, and 10% were used on Human Foreskin Fibroblasts, a combined cell line derived from two non-cancerous human foreskin samples in the presence of varying extracellular matrix (ECM), fibronectin,19 to observe any changes from these conditions. Indications from ingredients in this study suggest that increased β1 expression may yield a greater increase in G1 phase decreasing cellular proliferation with the increase in cola concentration.
HFF-1 cell culture and preparation
Human foreskin fibroblasts (HFF-1) from ATCC (Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Hyclone Ge Healthcare Life Sciences, Waltham, MA USA), 15% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 1% glutamax from Thermo Fisher Scientific (Waltham, MA USA) in a T75 flask, Thermo Fisher Scientific (Waltham, MA USA). Plastic bottled Coca-ColaTM (Coca-ColaTM, Atlanta, GA) was obtained on campus at California State Channel Islands University, from the vending machine near the laboratory in Sierra Hall. Cola was sterilized and decarbonized before dilution in HFF-1 Media at 0.5%, 1%, 1.5%, 2%, 4%, 6%, 8% and 10%. Cola pH was initially observed at 7.4 in a 1:1 ratio with HFF-1 media. Cola was vacuum filtered using Stericup Sterile Vacuum Filters (EMD Millipore, Temecula, CA). Cells were maintained in an incubator at 37°C and 5% CO2. HFF-1 cells were observed with a phase contrast microscope CKX41 from Olympus (Waltham, MA USA) for confluency. HFF-1 cells were washed with sterile phosphate buffer saline (PBS) Hyclone GE Healthcare Life Sciences (Waltham, MA USA). HFF-1 cells were trypsinized using 0.25% Trypsin-EDTA from Genclone (San Diego, CA, USA). Cells were observed under the phase contrast microscope during 10minute incubation. HFF-1 cells were neutralized with HFF-1 media. Mixture was transferred and centrifuged at 3000rpms in an Eppendorf centrifuge 5804R-15 AMP version (Hamburg, Germany). Supernatant was poured and suspended in HFF-1 media. An Auto Cellometer T4 (Nexcelom, Lawerence, MA) was used for all cell count calculations. Calcein-AM (Catalog #C3099, ThermoFisher, Waltham, MA) was used for staining, imaging and analyzation via Multimode Analysis Program, (Molecular Devices, Sunnyvale, CA). Fluorescent images were taken using Olympus IX71 inverted Fluorescent microscope from Olympus (Waltham, MA, USA) using a Cy3 filter at 10x magnification. All graphs and calculations were completed using Microsoft Excel. The statistical comparison between media and concentrations used was made by paired t-test (two-sample assuming unequal variances); P<0.05 was considered significant.
2D-cell proliferation assay
Cola was supplemented in HFF-1 media in the following concentrations: 0.5%, 1%, 1.5%, 2%, 4%, 6%, 8% and 10% from Coca-ColaTM (Los Angeles, CA, USA) and HFF-1 media. Cells were plated onto a 24-well plate, initially coated with 10µg/ml fibronectin at a density of 10,000 cells/ well and incubated at 37°C, 5% CO2. After 1hour, 3 days and 7 days of incubation wells were washed thrice, with PBS and incubated in the dark with calcein-AM. Fluorescent samples were quantified using FilterMax F5 Multi-Mode microplate reader from Molecular Devices.
Cell cycle analysis
HFF-1 cells were seeded at a density of 10,000cells/well in duplicates at 1.5%, 6% and 10% Cola supplemented conditions on 24 well plates initially coated with 10µg/ml fibronectin. HFF-1 with no initially coated fibronectin was also performed. Cells were harvested into 1.5mL Eppendorf tubes, centrifuged at 15,000rpm, and suspended into 1mL of PBS followed by another centrifugation at 5000rpm. After resuspension with 300μL PBS, 700μL 100% -20°C high grade ethanol was added. Cells were stored at -20°C until analysis. Cells were centrifuged at 200g for 5minutes and suspended with 200μL Guava Cell Cycle Reagent (Propidium Iodide solution), followed by a 30minute dark room incubation. Samples were analyzed using Guava EasyCyte flow cytometer from EMD Millipore (Hayward, CA, USA). Cell cycle analysis was analyzed at 5000 events per reading.
Integrin expression
HFF-1 cells were seeded onto a 24 well plate coated with 10μg/ml fibronectin at a density of 10,000cells/well in 1.5%, 6%, and 10% Cola supplemented media. Plates were incubated for 7 days. Post incubation, cells were washed with PBS, trypsinized, centrifuged and suspended in 1ml cold PBS using ice buckets. Cells were washed again with PBS and centrifuged at 15,000rpm for 5minutes. Cells were suspended in 800μl cold PBS. A 100μl was aliquoted per each media condition of 25,000cells. 2.5μl of either ⍺5 (Mouse Anti-Human CD49E Conjugate Monoclonal (Alpha 5 Antibody) EMD Millipore) or β1 (FITC Mouse Anti-Human CD29 (Beta 1 Antibody) Quantobio, Newbury Park, CA) antibodies were added and incubated on ice in the dark for 1hour. Two cold PBS and centrifugation wash steps were completed after incubation. Samples were analyzed using Millipore Guava easyCyte (EMD Millipore, Billerica, MA).
Initial adhesion
We first examined the initial adhesion, the rate at which cells first attach to the plate, on 24 well plates initially coated with fibronectin in the presence of Cola. Cells were seeded at 10,000cells per well in 0.5mL of regular media or Cola concentration media and observed after a 1hour incubation period. HFF-1 cells showed activation as the monolayer shown in Day0(s) (kept at relatively low density) and over time, losing their distinguishing round shape, and flatten down on the substratum leading to adhesion and cell proliferation.
N=9. Cola was stored at an average temperature based on human consumption (refrigerator) and then heated in a bath at 37°C before plating. Regular Media was hovering just above ~150%. 0.5% Cola decreased to ~125%. 1.0% had a slight increase to ~128% proliferation. The lowest percentage proliferation was observed at 1.5% Cola concentration at 100%. 2.0% Cola increased to ~160%. 4.0% Cola decreased slightly to ~150% proliferation. 6% Cola decreased to ~130%. 8% Cola shows the highest initial adhesion percentage at ~220%. 10% Cola decreased to ~150%.
We have compared the initial adhesion at day 0 (1hour incubation). The data compares all media types to the lowest initial adhesion percentage which was observed at 1.5% Cola concentration at Day 0 (1hour incubation). 1.5% Cola initial adhesion was set to 100% percent for comparison between media conditions. The two lowest initial adhesion percentages were observed at 1.5% and 6% Cola conditions. The highest initial adhesion observed was at 8% Cola concentration. All P-values marked with ‘*’ were considered statistically significant (P<0.05) when compared to HFF-1 cells cultured in regular HFF-1 media. Large overlapping standard deviations make data not statistically significant.
In conclusion, there is no observed trend from initial adhesion with the effect of Cola. Initial adhesion is the rate at which cells first attach to the plate.
Cellular proliferation
Figure 1 examines HFF-1 proliferation on 24 well plates initially coated with fibronectin in the presence of Cola. Cells were seeded at 10,000cells per well in 0.5mL of regular media or Cola concentration media and observed at Day 0, Day 3, and Day7. Cell proliferation results are a measure of HFF-1 proliferation on Day 0, Day 3, and Day 7 and a comparison of Day 3 and Day 7 proliferations to Day 0 readings of the same media conditions. Proliferation comparisons between media conditions are able to be made because of percentage representation of proliferation.
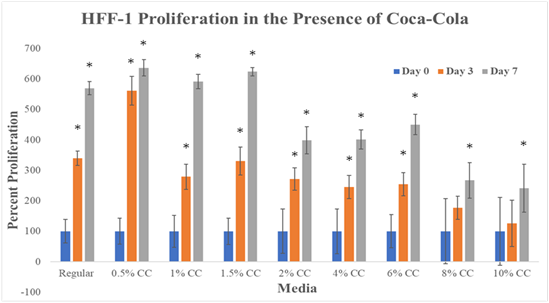
Figure 1 Cell Proliferation.
There is a decreasing trend observed with increase in the concentration of Cola in cell proliferation. Graph of HFF-1 cell proliferation on 10μg/mL fibronectin initially coated wells. Black brackets indicate standard deviation. N of 9. All P values marked with ‘*’ were P<0.05, considered statistically significant when compared to Regular Media. Day 3 proliferation shows a peak at 0.5% Cola and a negative correlation between proliferation and Cola concentration. The greatest rate of proliferation was seen in 0.5% Cola from Day 0 to Day 3. The lowest rate of proliferation was seen in 0.5% Cola from Day 3 to Day 7. Cells were seeded at 10,000 cells per well in 0.5mL of regular media or Cola concentration media. These cultures were incubated for 1 hour, 3 days, and 7 days, after which, were analyzed for their rate of proliferation by using a prepared calcein-AM suspension and the FilterMax F5 microplate reader.
Our data shows that HFF-1 cell proliferation across the Cola concentrations decrease after 1.5% Cola at day 3 and day 7. Day 3 regular media increased to ~340%. Day 7 regular media increased to ~570%. 0.5% Cola concentration had the highest cellular proliferation observed. Day 3 0.5% Cola increased to ~565%. Day 7 0.5% increased to ~620%. Cola concentrations; 1.0, 1.5, 2.0, 4.0, 6.0, 8.0, and 10% show a decreasing trend in cell proliferations. Day 3 1.0% Cola increased to ~290%, a 50% decrease from regular media. Day 7 1.0% increased to ~600%. Day 3 1.5% Cola increased to ~310%, a 30% decrease from regular media. Day 7 1.5% increased to ~610%.
Day 3 2.0% increased to 290%. Day 7 2.0% increased to 400%. Day 3 4.0% increased to ~280%. Day 7 4.0% increased to 400%. Day 3 6.0% increased to ~290%. Day 7 6.0% increased to ~450%. Day 3 8.0% increased to ~190%. Day 7 8.0% increased to ~270%. Day 3 10% increased to ~120%. Day 7 10% increased to ~200%.
We have compared results from Day 0 (1hour incubation), to Day 3, and Day 7. Day 0 proliferation percentages are presented as 100%. Our data shows that HFF-1 cell proliferation across the Cola concentrations decrease after 1.5% Cola at Day 3 and Day 7. There is a trend for an increase in the proliferation of HFF-1 in the presence of low concentrations (0.5%, 1.0%, and 1.5%) of Cola and decrease in proliferation in the presence of higher concentrations (2.0%, 4.0%, 6.0%, 8.0%, and 10.0%) of Cola on Day 7. Day 3 proliferation shows a peak at 0.5% Cola and a negative correlation between proliferation and Cola concentration. The greatest rate of proliferation was seen in 0.5% Cola from Day 0 to Day 3. The lowest rate of proliferation was seen in 0.5% Cola from Day 3 to Day 7. In conclusion, our results show a negative correlation between increasing concentration of Cola and HFF-1 proliferation. Cell proliferation results in an increase HFF-1 cell growth, and defined by the balance between cell divisions and cell loss through cell death or differentiation.
Cell morphology
Figure 2 shows calcein-AM staining of HFF-1 cell morphology at Day 0, 3, and 7. Calcein-AM is excited between the wavelengths of 495-515nm. It is transported (diffused) through the cellular membrane, passively, as a colorless reagent into live cells. While calcein-AM is a useful tool for visualizing cell morphology, extended incubation time of HFF-1 cells in calcein-AM is toxic. Cells were seeded at 10,000cells per well in 0.5mL of regular media or Cola concentration media and observed at Day 0, Day 3, and Day7. After entering the cell, intracellular esterase (hydrolase enzyme that splits esters into an acid and alcohol through hydrolysis) will remove the actomethoxy group, trapping inside the cell, emitting fluorescence through the cytoplasm with a low background. The dead cells do not carry the esterase.
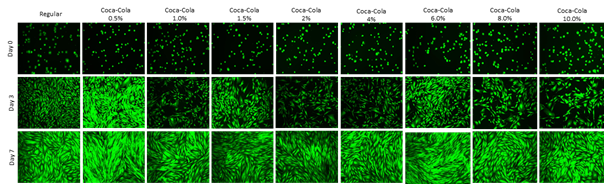
Figure 2 Cell Morphology.
Morphological analysis does not correlate with the HFF-1 Proliferation on day 3 but it correlates on day 7. Highest HFF-1 cell proliferation was observed on Cola 0.5% on Day 3 and Day 7. The calcein-AM staining of HFF-1 cells show the morphology from round appearance Day 0 to the spindle-like appearance by Day 3 and 7. The wells were initially coated with fibronectin 10μg/mL. HFF-1 media, 0.5%, and 1.0% look subjectively similar (based on appearance). 8% and 10% concentrations had notable large gaps between cell clusters in wells. Photos at 8%, and 10% appear more dimly lit and confirm cell proliferation decrease in Figure 2. Calcein-AM is excited between the wavelengths of 495-515nm. It is transported (diffused) through the cellular membrane, passively, as a colourless reagent into live cells. After entering the cell, intracellular esterase (hydrolase enzyme that splits esters into an acid and alcohol through hydrolysis) will remove the actomethoxy group, trapping inside the cell, emitting fluorescence through the cytoplasm with a low background. The dead cells do not carry the esterase.
Day 0 HFF-1 cells appear round and share similar size to one another. Day 0 images show similar cell concentration within the wells indicating that initial adhesion of HFF-1 cells was similar in all media conditions. Day 3 and 7 0.5% Cola photos show high amounts of cell density which reflects cell proliferation results from Figure 1. There is a trend from the images that the increasing Cola concentration decreases HFF-1 confluency at Day 3 and Day 7. The spindle-like appearance can be seen at Day 3 and Day 7. In conclusion, the Fluorescent images in Figure 2 confirm cell proliferation was highest in lower Cola concentrations compared to higher.
Cell cycle analysis
Figure 3 examines the cell cycle of HFF-1 in the presence of Cola concentration on initially coated fibronectin at Day 7. The analysis of these phases is based on the stoichiometric manner where the amount of PI stain (Propidium Iodide) is directly proportional to the amount of DNA within the cell. PI is an intercalating dye which binds to the DNA and double stranded RNA. Stained diploid cells that are analyzed via flow cytometry will show narrow readings of a distribution of fluorescent intensities. Figure 3 shows a quantitative histogram of the averages calculated for percent positive of HFF-1 phases.
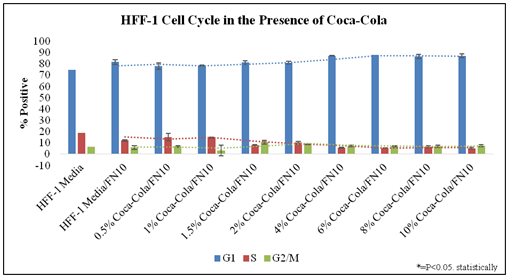
Figure 3 Cell Cycle Analysis.
There is an increase of G1 and decrease in G2/M Phase of Cell cycle analysis as Cola concentration increases. N= 2. HFF-1 cells were harvested, washed, and fixed, then on the day of use, were suspended in PI solution which binds easily in between the bases of the DNA with no preference.31 The percentage of cells in G1 phase, S phase and G2/M phase were detected using the Millipore Guava easyCyte flow cytometer. The cell cycle was analyzed in the presence of low and high concentrations of Cola. It is suggested by Figure 1 that in increased concentrations of Cola, there is an increase in the percentage of cells in the G1 phase, while there is also a decrease in the percentage of cell in the S phase. Day 3:G1 phase decreased between regular versus regular + fibronectin. S phase increased by 8% without fibronectin. The S phases of all conditions show no noticeable trend at Day 3. There is a slight decrease in G2/M phase as Cola concentration increases. S phase decreasing from 13% at Cola 1.5% to <10% at 6% Cola and <10% at Cola 10%. G2/M phase data points had no notable trends observed from the data. Day 7: G1 phase decreased between regular versus regular + fibronectin. S phase increased by 8% to 20% in HFF-1 cells coated without fibronectin. The S phases of all conditions showed no noticeable trend at Day 7. The S phases of all conditions showed no noticeable trend at Day 7. G2/M phase data points had notable trends observed.
There is a trend for lower G1-phase presence at low concentrations (0.5% and 1.0%) of Cola when compared to Regular media in 10μg/mL fibronectin initially coated wells. There is a trend for higher G1-phase presence at high concentrations (4.0%, 6.0%, 8.0%, and 10.0%) of Cola when compared to Regular media in 10μg/mL fibronectin initially coated wells. There is a trend for decreased S-phase presence as Cola concentrations increase when compared to Regular media in 10μg/mL fibronectin initially coated wells. G2/M phase data points had notable wide standard variations with no trend observed from the data in the presence of Cola.
In conclusion, G1 phase increases as Cola concentration increases. A trend can be observed of the S phase decreasing from 13% at Cola 0.5% to <10% at 10% Cola. G2/M phase data points had notable wide standard variations with no trend observed from the data.
FACS analysis
The antibodies used in FACS analysis for integrin expression binds to the minor grooves of the DNA helix and does not bind to RNA. Antibodies consist of a variable region (Fab portion) that will bind to the epitope (fibronectin or collagen) and the constant region when specified.20
Fluorescent Activated Cell Sorting (FACS) results using Guava Incyte software of HFF-1 cells after 7 day of incubation in the presence of 1.5%, 6%, and 10% Cola and HFF-1 growth media on wells initially coated with 10μg/mL fibronectin. The vertical black line depicted in Figure 4A-4H was used to determine shifts in integrin expression. Figure 4A & 4B establishes baseline for integrin expression activity.
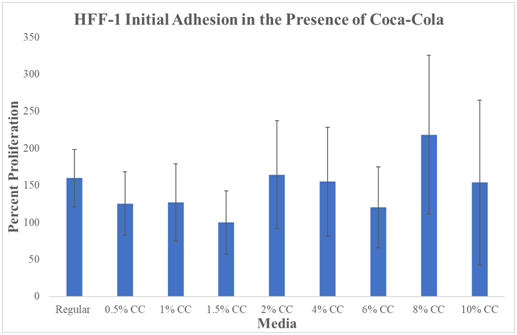
Figure 4 Integrin Expression.
Increase in expression of α5β1 integrin in Regular, 1.5%, 6%, and 10% Cola. Fluorescent Activated Cell Sorting (FACS) results using Guava Incyte software of HFF-1 cells after 7-day of incubation in the presence of 1.5% Cola, 6% Cola and HFF-1 growth media on wells initially coated with 10μg/mL fibronectin. The black line depicted in Figure 4A-4H was used to determine shifts in integrin expression. Figure 2A & 2B establishes baseline for integrin expression activity. There is no observed trend in α5 or β1 expression with any conditions compared to regular. Black peaks indicate no antibodies added. High background was noted. N=2
Figures 4C-4H shows no change in the expression of α5 or β1 integrin. Black peaks indicate no antibodies added. High background was noted. Black peaks indicate no antibodies added. High background was noted. In conclusion, no change in α5 or β1 integrin expression by HFF-1 cells in the presence of Cola was observed.
Soft drinks have been a target for their high sugar content and otherwise unknown ingredients, in their contribution to risk factors in human diseases.6 Fibroblasts rely on an intricate balance of cellular mechanisms such as proliferation, focal adhesion, migration, and apoptosis for survival. To maintain proper functioning of a tissue, cells must be able to communicate efficiently to neighboring and far reaching cells.15 Human foreskin fibroblasts migrate and attach themselves to ECM proteins by focal adhesion to fibronectin. This is done through integrin binding α5β1 to the RGD (Arg-Gly-Asp) cell-binding domain.21,22 As conformational change occurs, integrin clustering and exposed fibronectin binds to the sites promoting fibronectin-fibronectin interaction, critical for initial adhesion and wound healing.23 During wound repair, this allows for the arrangement for new granular tissue, producing and renewing the extracellular matrix.24,25
Initial adhesion
An inverse relationship between HFF-1 initial adhesion and HFF- 1 proliferation was expected. An increased attachment rate to the surface would indicate a stronger adherence and a lesser detachability from the HFF-1 cells.26 Therefore, an increase in initial adhesion would yield a lesser proliferation however; no statistical difference in initial adhesion was observed when HFF-1 cells were incubated for 1hour in the presence of Cola and regular media. There is a trend that low concentrations of Cola have lower initial adhesion than higher concentrations of Cola.
The ability of fibronectin to induce organization of important proteins to promote adhesion-dependent cell-growth could be the reason behind fibroblast proliferation in regular media.27‒30 There was more cell proliferation of fibroblasts in the presence of high levels of Cola, such as 0.5% Cola Increased acidity may have inactivated fibroblast growth factor but the pH of the media remained normal even in 1:1 ratio of Cola to HFF-1 media.31 High fructose corn syrup is about 55% fructose and can have adverse effects on cells due to high concentration of a minor sugar.32,33
Fibroblasts migrate on 2-dimensional surfaces using actin-rich extensions at the ends also known as lamellipodia. Under the plasma membrane, the actin filaments are polarized. This enables the exertive force causing opposing tension, pushing back into itself and through integrin mediated adhesion paired with cytoskeleton to a substrate, allows these forces to contract.34‒37 Therefore, increased adhesion could cause a delayed detachment and retraction of the lagging portion of the cell. In contrast, both collagen and fibronectin increase migration of fibroblast cells.28,38-40 Having both fibronectin and Cola together show that the possible increased initial adhesion effect of high concentrations of Cola is greater than the decreased initial adhesion effect that fibronectin has on HFF-1 cells.
Cell proliferation
Calcein-AM stains were used over other assays such as MTT due to the cellular retention.41 While MTT are robust assays, ascorbic acid, found in soft drinks can alter these results. Due to the unknown knowledge of Cola on cellular proliferation, if there was over confluence paired with an MTT assay, inability to record accurate readings.42,43 A significant decrease in HFF-1 proliferation incubated in the presence of increasing amounts of Cola is demonstrated in Figure 1. This suggests that because there is an increase in the initial adhesion, therefore it requires more energy for these cells to detach, go through DNA duplication, and cell division. Phosphoric acid could be blocking necessary divalent cations such as Mg2+, Mn2+, and Ca2+ used in integrin receptors for binding ligands.44 Cola at lower concentrations of 0.5% showed higher cell proliferation and lower cellular adhesion. It has been found that carbonated soft drink consumption is associated with shortening of telomere length critical in cell viability and chromosomal stability.45 There are multiple ingredients within Cola that affects cells. Increased caffeine levels reduce collagen expression in the liver and therefore reduce levels of TGFβ reducing fibrosis in epithelial cells.46 Fibroblasts incubated in various caffeine concentration express β1-integrins and insulin growth like receptors which contribute to the inhibition of collagen biosynthesis.33 4-methylimidazole was a coloring agent that since has been removed due to its effect on cellular proliferation, including DNA breaking and DNA fragmentation.47,20 Sugar substitutes influence angiogenesis, inducing regenerative cytokine production, activating MAPKs, enhancing IL-6 and VEGF. This result in angiogenesis, an important factor in wound healing and tumors.48
Cell cycle analysis
Cell Cycle analysis showed that higher number of cell arrest in G1 phase and lower in S and G2/M phase. G1 phase arrest occurs when there is an increase in the number of cells that are expanding in size but are prevented from duplicating their chromosomes.49 A rapid accumulation of G1 phase cells before S phase progression may be a strategy for safety in cell proliferation due to a signal from vascular injury.50 Fibroblasts arrested at the G1-S transition lack regulator kinases observed in human cancers.51 Other studies have shown that S phase fibroblasts arrest at G0-G1 phase by interaction with collagen type I.52 S phase appeared to be decreasing as Cola concentration increased. The transition from proliferation to senescence is driven by the endogenous and exogenous stress factors of increased concentration. Senescent cells remain metabolically operative but exit the cell cycle and stop proliferation, resulting in a decreased incidence of cancer. Caffeine amounts at 1mM on epithelial cells have shown decreased the G2 arrest.53,54 Coca extract was shown to have an increasing trend in the appearance of the hypodiploid sub-G1/G0 phase, with increasing Cocoa procyanidin-rich extract concentration, indicative of DNA damage, and cell death.55 The ERK ½pathway impairs MAP kinase; therefore, we see a decrease in S phase.56 Due to the decrease in DNA synthesis this may be a trend between proliferation and cell cycle data. High Cola concentrations show an increased presence of cells in the G1 phase and a lower proliferation showing that cells are surviving but proliferating which generate a low proliferation rate.
Integrin expression
Figure 5 showed initial adhesion of HFF-1 cells to fibronectin coated surfaces increased in the presence of higher concentrations of Cola, suggesting an increase in α5β1 integrin expression, a receptor specific to fibronectin binding. These interactions show that cell adhesion, through integrin expression results in morphology change in cell shape, growth rate, and differentiation allowing the ability to thrive in different environments.57-59 Fibronectin interact with cells mostly via integrins αvβ3 and α5β1 during tissue repair process.3,60 However there were no observed changes in the expression of α5β1 integrin in Cola concentrations 1.5%, 6.0%, and 10.0%.
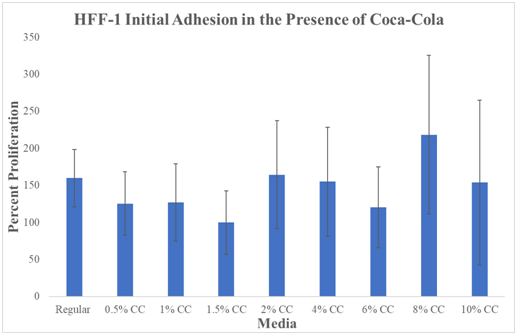
Figure 5 Initial Adhesion.
There is no statistically significant difference between HFF-1 initial adhesion in Cola or regular media. Graph of HFF-1 initial adhesion on 10μg/mL fibronectin initially coated wells. Black brackets indicate standard deviation. N=9. All P values marked with ‘*’ were P<0.05, considered statistically significant when compared to Regular Media. Cola was stored at an average temperature based on human consumption (refrigerator) and then heated in a bath at 37°C before plating. Comparison is from each result from regular. The data compares all media types to the lowest initial adhesion percentage which was observed at 1.5% Cola concentration at Day 0 (1hour incubation). 1.5% Cola initial adhesion was set to 100% percent for comparison between media conditions. The two lowest initial adhesion percentages were observed at 1.5% and 6% Cola conditions. The highest initial adhesion observed was at 8% Cola concentration. Cells were seeded at 10,000 cells per well in 0.5mL of regular media or Cola concentration media. There is a trend that high concentrations of Cola generate higher levels of initial adhesion than low concentrations of Cola.
It has been shown in recent articles that increase in β1 integrin expression can be due to high glucose amounts which coordinate an increase in levels of mRNA encoding fibronectin and its corresponding receptor.61 Therefore, determination of integrin specificity with specific ECM domains is important in maintaining cell homeostasis, host defense and tissue maintenance.62
Further, binding of integrins to the ECM facilitates cell attachment via a signaling cascade that lead to fibrillogenesis63 Fibroblasts cultured in fibronectin require ubiquitination of integrin controls migration through lysosomal degradation via the endosomal sorting complex required for transport.64
α5β1 Integrin binding serve as a mode for both internal and external cell signaling, and promotes cell migration, cell survival and cell proliferation.65 Integrin β1 subunit mediates the attachment of fibroblasts to collagen type I and fibronectin, suggesting that integrin β1 would be a key factor in the focal adhesion, cell migration and cell proliferation when in the abundance of fibronectin.21,66 Due to the increase in expression of these surface proteins, there is an increased connection to fibronectin-rich environments, and will therefore have an increased rate of initial adhesion in the presence of increased Cola concentrations.
An increase in focal adhesion of HFF-1 cells to fibronectin in the presence of increased concentrations of Cola suggests that there could be an increase in the expression and interaction of α5β1 integrins on the cell surface of HFF-1 cells to fibronectin rich ECM environments. However further test should be completed.
An increase in these interactions suggest that the tension between integrins to a fibronectin surface are highly increased, therefore not allowing cells to be able to detach from the surface to circularize to proliferate.57 Therefore, Cola beverage has an increase in initial adhesion of fibroblasts, yielding to a decreased rate of HFF-1 proliferation. Cell Cycle analysis confirms this revealing an increase in the G1 phase and decrease in the S phase. Visual confirmation and cell morphology also verifies the effect with increase in Cola concentration.
Fibroblast can secrete their own extracellular matrix proteins to restructure their surrounding environment.49 Collagen and fibronectin are excreted by HFF-1 cells dependent on their surrounding environment. As seen in Figure 1, HFF-1 cells grown in the presence of a fibronectin-rich environment excrete high amounts of collagen This is observed because these cells were introduced into an environment where they were in need of collagen, and because there was none provided for them, they secreted their own to survive. Fibroblasts are key initiators in the wound healing process, and the fact that they can excrete their own extracellular matrix proteins, is the key factor why they play such an important role. In future studies, the secretion of fibronectin and collagen should be examined in the presence of different concentrations of Cola, to better understand this beverage’s impact on fibroblasts’ role in the wound healing process.
We would like to thank our lab partners: Brianna Bucci, Atherton Kniseley, Nikita Vaghasia, and Kyle Eckhart, we could not have done any of this without them; and Dr. Bill Tawil and Melissa McCoy for supporting our group through our experiments and encouraging our research.
The article was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
California State University, Channel Islands, Camarillo, CA. Extended Education-CSUCI
Author declares that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.