eISSN: 2574-9838


Short Communication Volume 8 Issue 1
Department of Advanced Polymeric Nanostructured Materials Engineering, Toyota Technological Institute, Japan
Correspondence:
Received: February 06, 2023 | Published: February 17, 2023
Citation: Okamoto Y, Okamoto M. Biocomposites composed of cartilage and natural rubber latex. Int Phys Med Rehab J. 2023;8(1):40-41. DOI: 10.15406/ipmrj.2023.08.00331
We have successfully fabricated the cartilage/natural rubber latex (NRL) biocomposites via human mesenchymal stem cells (hMSCs) spheroid under hypoxic condition. It was revealed that NRL nanoparticles acted as the main component, providing surface heterogeneity for the spheroids and resulting in a mechanically stable structure with a modulus two times higher compared to controls. The formation of the cartilage/NRL nanoparticles biocomposite results in having a stiff property was demonstrated. The gene expression levels of SRY-box 9 (SOX9), aggrecan and type II collagen (Col-II), and formation of glycosaminoglycan’s were higher in spheroids under hypoxic conditions, and administration of NRL nanoparticles enhanced chondrogenic differentiation is successfully induced. The promise of NRL nanoparticles for well-controlled chondrogenic differentiation and mechanically stable cartilage tissue was demonstrated.
Keywords: natural rubber latex, hMSC, biocomposites, chondrogenesis, spheroid, mechanical properties, normoxia
NRL, natural rubber latex; hMSCs, human mesenchymal stem cells; SOX9, SRY-box 9; Col-II, collagen type-II; HIF-1&α;, hypoxia-inducible factor 1&α
In tissue engineering and regenerative medicine chondrogenesis has been a very challenging topic due to the difficulty of self-healing of cartilage damage.1,2 Previous studies have reported that damage of hyaline cartilage caused by trauma is repaired with fibrocartilage, which is inferior in mechanical functions as compared to that of hyaline cartilage.3 For this reason, the regenerative tissue for articular hyaline cartilages is required to provide the mechanical function for withstanding the high pressure.
Human mesenchymal stem cells (hMSCs) have shown to have the potential to differentiate into several cell types including chondrocytes and excellent proliferation property.4 Intravenous injection is the most common method of administration of hMSCs, but a large part of it is trapped in the lungs, and only a part of it reaches the injured part, so it cannot be applied to tissues without blood vessels such as cartilage. Therefore, in the case of regenerative medicine, an approach of transplanting hMSCs that has induced differentiation into the target organ is being investigated.5 However, to date, the sequential effect of hMSC differentiation into chondrocytes and providing a mechanically stable structure into cartilage tissue has not been reported.
We have reported some promising results on regenerative tissues of natural rubber latex (NRL) in our previous papers.6,7 In this paper, we have used hMSCs to examine in vitro chondrogenesis in the presence of NRL nanoparticles to examine the promising approaches. In addition, with administration of NRL, chondrogenesis of hMSCs in hypoxic condition was investigated considering that chondrogenic differentiation of hMSCs is promoted by hypoxic condition. We fabricated the biocomposites of cartilage/NRL under hypoxic condition and examined that the NRL nanoparticles are to be a potential loading component for cartilage tissue repair.
Materials: The NRL solution sample (solid content of 35.3wt.%) added ammonia (0.5wt.%) and preservatives (BIT: 1,2-benzisothiazolin-3-one) (0.15wt.%) as additives.7
Gene expression analysis: In order to form spheroids, hMSCs (Takara Bio) were seeded onto EZsphere® 96-well plate (Iwaki) and incubated in Mesenchymal Stem Cell Growth Medium 2 (Takara Bio) at 37 °C under 5% CO2 for 24h. After formation of spheroids, culture medium was changed to Chondrogenic Differentiation Medium (Takara Bio) containing NRL (0.32mg/mL) were administrated to each well. After 3 days, spheroids were transferred to microtubes at concentrations of 2×105 cells each. The spheroids were harvested at different time points (day 3, 7, 14 and 21) in normoxia (5% CO2, 20% O2) and hypoxia (5% CO2, 1% O2) at 37 °C. The medium was changed every 3days. Gene expression analysis of hMSC was performed using Real-time polymerase chain reaction (RT-PCR) (Light Cycler® 96: Roche) according to the protocol of Transcriptor Universal cDNA Master (Roche). Statistical analysis was performed using Tukey-Kramer’ post-hoc testing.
Compression test: After changing the culture medium to chondrogenic medium containing NRL (0.32mg/mL) and culturing for 3days, the cells were transferred to conicaltubes (Proteo-Sava® SS, Sumitomo Bakelite) at a concentration of 2×106 each and cultured in hypoxia for 21days. The stress–strain curve was created using a microautograph (MST-I Shimadzu Co.) at a strain rate of 120µm/min. Considering that the samples are elastic bodies, the elastic modulus was calculated from the stress change in the section where the strain was 0.1–0.3.
Gene expression analysis
The hypoxia-inducible factor 1α (HIF-1α) level under hypoxia is markedly increased in hMSCs incubated at 14 days as compared with that of normoxia (Figure 1). In hypoxia the expression of SOX9 gradually increases throughout 21days of culture (Figure 2). However, no statistical difference between spheroids with and without NRL is observed. The expression of Aggrecan is over 6-fold at 21days in comparison to control. This suggests that the cartilage cultured with NRL nanoparticle produce a large amount of aggrecans. The collagen type-II (Col-II) is up-regulated at day14. The expression level in NRL-loaded spheroids is more than 4-fold in comparison with the control. The expression level of SRY-box 9 (SOX9), aggrecan and Col-II are higher in the spheroid, indicating that chondrogenic differentiation is successfully induced with the administration of NRL nanoparticles under hypoxic condition.8
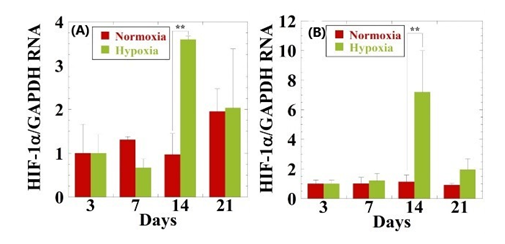
Figure 1 Effect of oxygen concentrations on gene expression of HIF-1α for (A) hMSCs and (B) hMSCs cultured with NRL nanoparticles of 0.32mg/mL at different time interval (3 to 21days). Data were normalized to the data at day 3 and were expressed as mean ±S.D. (n=3). **: p < 0.01. Reproduced with permission from.8 © 2020, Taylor & Francis Group.
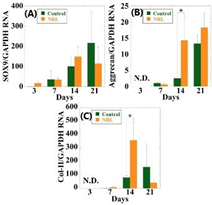
Figure 2 RT-PCR analysis of the (A) SOX9 and (B) Aggrecan, (c) Col-II expressions for hMSCs and hMSCs cultured with NRL nanoparticles of 0.32mg/mL at different time interval (3 to 21days) in hypoxia. Data were normalized to those of control at day 3 for (a) or at day 7 for (b) and (c) and were expressed as mean ±S.D. (n=3). *: p<0.05. N.D. indicates data were not detected.8 © 2020, Taylor & Francis Group.
The stress–strain curve of spheroids was obtained by a compression test using the microautograph (Figure 3). The elastic modulus obtained from the stress-strain curve was 67.7kPa, and it was confirmed that the elastic modulus was more than doubled in comparison with that of control (33.6kPa). By loading NRL, the cartilage tissue with excellent mechanical strength was formed as a bulk.
Gene expression analysis revealed that NRL promotes the expression of cartilage-related genes in hypoxia. In the compression test, the elastic modulus was significantly improved. The elastic modulus of the spheroid cultured with NRL was about twice as high as that of control, indicating that NRL has an excellent ability as a reinforcing material for cartilage. The results showed promise of the NRL nanoparticles for well-controlled chondrogenic differentiation and mechanically stable cartilage.
This work was supported by the KAKENHI of the Ministry of education, Sports, science and Technology, Japan.
Contributed to the initiating idea and performed most of the experiments: Okamoto Y. and Okamoto M. Supervised research: Okamoto M. The manuscript was written through contributions of all authors.
Author declares that there is no conflicts of interests.

©2023 Okamoto, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.