eISSN: 2574-9838


Objective: To identify the relationship between the Psychomotor Performance and the Intelligence Quotient in preschoolers diagnosed with Congenital hypothyroidism (CH).
Methods: Transversal study with 58 preschoolers diagnosed with CH. Picq and Vayer test (PV) and Wechsler preschool and primary scale of intelligence (WPPSI) were used. Pearson's correlation coefficient and analysis of variance were estimated.
Results: Body schema (BS) from the PV test showed the highest statistically significant correlation with global IQ from the WPPSI (r=0.410); verbal IQ (VIQ) with Language (LE) (r=0.479); and performance IQ (PIQ) with BS (r=0.444). Components from the PV test showing significant correlations with verbal sub-scale of WPPSI. Projective drawing correlated to 4 of the 5 sub-scales assessed by the Performance sub-scale fo WPPSI (r 0.267 to 0.465).
Conclusion: Psychomotor development in preschoolers is the major predictor of the performance functions of children with hypothyroidism.
Keywords: psychomotricity, intelligence quotient of preschoolers
Human action or motricity can only be conceived as psychomotricity when the motor component is dynamically inter-related to the cognitive component; it is this neuropsychomotor interaction which provides the intrinsic and unique feature in its evolutionary integration and the adaptation of the species.1
The term Psychomotricity arises from the need to relate two elements: body and mind, in a time where the basic role of the mind was favored over the body, which was reduced to a simple support or structure of human beings. This dissociation found its first oppositions in the work of Dupré in 1907, who considered the relation between mental disabilities and motor disorders, describing the first specific clinical picture: motor weakness. According to this, every feeble-minded shows alterations and delays in their motor skills. Later on, Wallon (1925) considered the importance of body movement for the development of child's mind and for the construction of the body schema and image, where psychism and motor skills represent the expression of relations between the subject and their environment. These ideas suggested new relationships between mind and body. From these contributions, the possibility to explain motor disorders from the presence of cognitive development alterations was considered, thus evidencing the existing correlation between mind and motor activities.2,3
Picq and Vayer conceived psychomotor education as the "pedagogical and psychological action using the physical education to normalize or enhance child's behavior". Considering the use of the body as a mediator to address the human motor act, with the goal of turning it into an adaptive resource for the interaction of the subject and their environment.4
The work of Jean Piaget permits the development of intelligence and its relation to psychomotricity. He established that the activity and sensory-motor experiences are crucial for the development of cognitive skills.4
Movement is studied in psychomotricity as a development factor; the individual interacts with their environment through both physical and social actions.3 This function of relating with the world sets up the perceptive capabilities, the structure of space-time, the symbolization capabilities, and the regulation of their own actions. Thus, in the first years of life, the ability to perform motor acts is a major predictive factor of the later cognitive function.5–9
Preschool is extremely important for development, as it is in this period of time when the child masters a series of motor abilities that will set their overall maturity, integrating both the intellectual and affective dimensions.10 Motor activity is crucial because it enables the child to continue with the organization of their body image, in the environment where they develops, and to serve as a starting point for the organization of the motor skills being developed, regarding to their perceptive analysis abilities.11
After the age of three or four years old, movements become more harmonious, there is better control on impulses, and postural control, static balance, and coordination of movements are enhanced, thus allowing an increased autonomy. Additionally, lateral dominance is defined. This laterality helps to build the body schema and to consider the symmetry of the body, as well as to establish the spatial structure. When the child perceives the central axis of their body, they will be able to organize every element in their environment regarding to this axis.12 Practically all functions are lateralized, e.g., comprehensive and expressive language, intonation and linguistic creativity; spatial perception; faces and shapes recognition; human voice recognition, verbal and non-verbal memory; and literacy, among others.11
Planinšec and Pisot13 conducted research with adolescents and found that those with average intelligence performed better on tasks involving coordination, those who had a low IQ. They reported that the visual-motor integration and bimanual coordination are important elements of movement that are linked to the inteligencia.13 Recently, a study showed results that stronger associations between motor and intellectual functions; they were in task execution visomotoras.14
The speed of movements when performing any activity has also been described as an essential component for the efficiency of intellectual processes and the resolution of problems, as well as structuration, regulation and motor control.15,16 Children requiring less time to perform tasks involving speed, showed better scores in those tasks requiring verbal-logical reasoning, social situations comprehension, and a tendency in those tasks related to defining concepts through verbal signs and symbols and the verbal IQ; as well as those tasks requiring respiratory control, visuomotor coordination, and body schema.17–19
On the other hand, it is well known that thyroid hormones are necessary to achieve an optimal development in children, so its deficiency or malfunction determines physical, motor and intellectual development delays. Some studies have described a number of alterations in children with Congenital Hypothyroidism (CH) for postural control, fine oculomotor coordination, psychomotor and visuospatial skills, auditory discrimination, language, and attention.20,21 Thus, it is very important to investigate how these processes occur in children with an endocrine disorder, such as CH.
CH is the lack of thyroid hormone at birth; it is the most common preventable cause of intellectual disability among endocrine diseases.22 Its incidence in the US and Mexico is reported to be 1 in 2,372, and 1 in 2,000 newborns, respectively.22,23 Between the years 2000 and 2004, a total of 2,777,292 newborns were studied in the Instituto Mexicano del Seguro Social (IMSS, Mexican Institute of Social Security). The domestic incidence rate was found to be 4.3 in 10,000 (1 in 2,325) newborns, and Nuevo León was the state showing the maximum incidence rate, i.e., 7.8 in 10,000 (1 in 1,282) newborns.24 It is more frequent in Hispanics and native Americans than in black people, and it is also predominant in women (2:1). Children with Down syndrome are at greater risk.25
The age at treatment initiation, initial dose and the time required to achieve and maintain desired levels of thyroxine (T4), and thyroid-stimulating hormone (TSH), all play a major role to avoid long term consequences.26,27 Thus, screening programs have been created worldwide for the early detection of this and other ailments. However, replacement therapy initiation is still late in Mexico.28 Vela-Amieva et al.23 reported that during 2001 and 2002, the average age at treatment initiation was 25.19 days later than the recommended age (30 days).29 The need to establish the optimal time for treatment initiation has been shown. The American Academy of Pediatrics and the American Thyroid Association (2006) have set the goal for the ideal age for treatment initiation at 15 days of life.25
Early start of hormone replacement therapy is not conditionally sufficient to prevent the risk of developmental disorders, since some types of hypothyroidism involve hypothyroxinemia during life inside the uterus, resulting in bone age delay. However, it is suggested that a younger age at treatment initiation is related to a better neurological outcome.30
The goal of this study was to identify the relation between Psychomotor Performance assessed by means of the Psychomotor Exam of Picq and Vayer and the Intelligence Quotient from the WPSSI in preschoolers diagnosed with CH.
A total of 58 children diagnosed with Congenital Hypothyroidism receiving hormone replacement therapy by the Endocrinology Services in the Neurodevelopmental Follow-Up Laboratory were included in this study. Children were assessed from 4 to 6 years of age. Before enrolling patients in the study, their parents were asked to provide their written informed consent. Late therapy initiation was regarded as hormone administration after 30 days old.
The Picq and Vayer test (1977) was used; this test yields a psychomotor profile through the evaluation of several psychomotor functions: Oculo-Manual coordination (consists of the action of hands, or any other part of the body, performed in coordination with the eyes); dynamic coordination (it requires the ability to synchronize different parts of the body); postural control (its purpose is to maintain posture and to perform movements); balance (it is the ability to compensate the effect of gravity and maintain the body at a desired position); body control (it compiles a set of static and dynamic abilities comprising postural and tonic adjustments, and allows a balanced relationship between the body, the gravitational force, and the support surface); organization of the left-right spatial orientation (ability to estimate spatial distances and to perform motorize adjustments necessary to go over them, using the abilities of spatial analysis, distance and direction processing, and motor planning); perceptive organization (organization and structuration of sensory information from both their own body and the environment, and its integration into perceptive schemes resulting in the projective reality); space-time structuring (ability to locate themself with respect to themself and others, as well as the ability to relate facts in a time period); lateralization (it is the preference for the more frequent and effective use of one half of the brain over the other); rhythm appreciation (ability to synchronize movements to a rhythm, first with the hands, and then with locomotor movements); drawing (used to examine personality, degree of psychomotor maturity and body image); speed (ability to perform simultaneous and synchronized movements with limbs); simultaneous movements (ability to perform synchronized and simultaneous movements with different directionality); body concept (individual's ability to conceive an inside image of themself).31
The WPPSI scale was used for the assessment of the Intelligence Quotient in children from 4 to 6.5 years old. This scale is comprised by two scales: verbal and performance. It yields three Intelligence Quotients: Global (GIQ), Verbal (VIQ), and Performance (PIQ).32
Each patient was assessed individually in a single session of 60-90 minutes. Criteria established by the authors were used to estimate the scores from the instrument. For all three coefficients of the WPPSI, =100 and SD=15 were set according to the manual, and X=10 and SD=3 were set for the normalized scores of the sub-scales. As for the Picq and Vayer test, data normalized by age were used, and =100 and SD=15 were set. Descriptive analysis was carried out on the data from variables and Pearson's correlation coefficients were estimated for the correlation between the scores from the Picq and Vayer test and the normalized scores from the WPPSI. Additionally, an ANOVA was carried out for genre and type of CH, with the results from the sub-scales.
58 children (48 female and 15 male) with ages in the range from 4 to 6 years old (=4.7+0.51 years) were included in this study: 4 years old (17 children), 5 years old (34 children), and 6 years old (7 children). Sample demographics are show in Table 1.
Mean |
SD |
|
Child's age (years). |
4.7 |
0.51 |
Age at treatment initiation (days) |
44 |
± 15 |
n |
Prob |
|
Sex |
||
Female |
43 |
0.741 |
Male |
15 |
0.259 |
Type of CH |
||
Athyrosis |
27 |
0.465 |
Ectopic |
31 |
0.535 |
Mother's years of education |
8.6 |
2.6 |
Table 1 Study sample demographics
From the statistical analysis of the VIQ, PIQ, and GIQ by Genre, Type of CH, Age at TX initiation, and Mother's years of education, we found statistically significant differences for all three IQs assessed in the WPPSI by Type of CH (approximately 10 points) and only in the VIQ and GIQ by Age at TX initiation. Late TX initiation showed the lowest score regarding to Early and Intermediate initiation (differences were greater than 13 points). Genre and mother's years of education were not statistically significant Table 2.
Verbal IQ |
Performance IQ |
Global IQ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
Mean |
SD |
p |
Mean |
SD |
p |
Mean |
SD |
p |
|
Total population |
96,4 |
± 12,7 |
99,1 |
±15,2 |
97,5 |
±13,4 |
|||
Genre |
|||||||||
Female |
96,2 |
± 11,3 |
NS |
100 |
±14,7 |
NS |
97,8 |
±12,6 |
NS |
Male |
97,2 |
±16,6 |
96,3 |
±16,8 |
96,5 |
±15,9 |
|||
Type of CH |
|||||||||
Athyrosis |
90,5 |
± 13,4 |
0.0005 |
94,1 |
±15,2 |
0.0181 |
91,4 |
±14 |
0.0008 |
Ectopic |
101,6 |
± 9,6 |
103,4 |
±14 |
102,8 |
±10,5 |
|||
Age at TX initiation. |
|||||||||
Early |
101,4 |
± 10,4 |
97,1 |
±14,9 |
99,2 |
±11,6 |
|||
Intermediate |
97,5 |
± 12,4 |
0.0182* |
101,3 |
±15,3 |
NS |
99,3 |
±13,1 |
0.0353* |
Late |
85,4 |
± 11,8 |
89,9 |
±12,9 |
86,3 |
±13,1 |
|||
Mother's years of education |
|||||||||
<=6 |
95,1 |
±14,4 |
NS |
95,6 |
±13,7 |
NS |
94,8 |
±14,2 |
NS |
6 |
96,8 |
±12,4 |
100 |
±15,6 |
98,2 |
±13,3 |
|||
Table 2 IQ differences by Genre, Type of CH, Age at treatment initiation and Mother's years of education
The ANOVA showed no statistically significant differences, by Genre, in the scores of the sub-scales of the Verbal scale of the WPPSI. A small trend was observed in Similarities, where boys scored higher than girls, and Comprehension and Sentences, where boys scored lower than girls. As for the Performance scale, sub-scales showing statistically significant differences (p<0.005) by Genre were Animal house and Copying geometric design, where girls scored higher than boys.
The ANOVA showed statistically significant differences, by Type of CH, in the scores of all the sub-scales of the Verbal scale of the WPPSI; the Athyrosis group scored lower than the Ectopia group (Figure 1).
The ANOVA showed statistically significant differences, by Type of CH, in the scores of the Animal house, Copying geometric design, Picture completion, and Mazes sub-scales of the Performance scale of the WPPSI. The biggest delays were observed in the Athyrosis group (Figure 2).
The ANOVA showed no statistically significant differences, by Genre, in the scores of the scales assessed by the Picq and Vayer test. However, there were statistically significant differences, by Type of CH, in Body control and Perceptive organization (Figure 3).
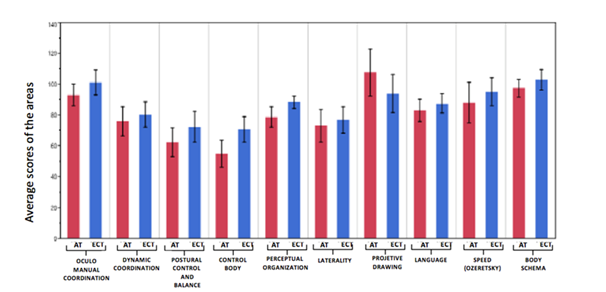
Figure 3ANOVA for the means of the Scales assessed by the picq and Vayer test by type of hypothyroidism.
Correlation analysis showed statistically significant associations between the Global IQ and seven of the 10 scales of the Picq and Vayer test; the highest correlations were observed with Body schema (BS) (r=0.410), Language (LE) (r=0.3894), and Body control (BC) (r=0.3893). Verbal IQ (VIQ) showed statistically significant associations with four scales of the Picq and Vayer test; the highest correlation was observed with LE (r=0.479). The Performance IQ (PIQ) was correlated to five scales, the most important were with BS (r=0.444), and PD (r=0.404) (Table 3).
Picq and vayer test scales |
Intelligence quotient |
||
|---|---|---|---|
Verbal IQ (VIQ) |
Performance IQ (PIQ) |
Global IQ (GIQ) |
|
Oculo-Manual Coordination (OMC) |
- |
0.367 |
0.276 |
Dynamic Coordination (DC) |
- |
- |
0.273 |
Postural Control And Balance (PCB) |
0.379 |
0.279 |
0.374 |
Body Control (BC) |
0.352 |
0.331 |
0.389 |
Projective Drawing (PD) |
- |
0.404 |
0.374 |
Language (LE) |
0.479 |
- |
0.389 |
Body Schema (BS) |
0.274 |
0.444 |
0.41 |
Table 3 Correlation coefficients between the scales of the Picq and Vayer test and the Intelligence quotients of the WPPSI*
*Only statistically significant correlation coefficients are shown (p<0.05).
When the Picq and Vayer test is correlated to the WPPSI, we observed the following results. Components showing statistically significant correlations with several sub-scales from the Verbal scale of the WPSSI were LE, PBC, and BC. The LE component was correlated to 6 assessed sub-scales (Vocabulary, Comprehension, Sentences, Information, and Similarities), while the PBC and BC components were correlated to four and three assessed sub-scales, respectively. Vocabulary was the sub-scale showing the highest correlation with LE. In the Performance scale, PD was correlated to 4 of the 5 sub-scales assessed by the WPPSI, and the correlation coefficients ranged from 0.267 to 0.465; OMC, BS, and BC were correlated to 3 sub-scales. The sub-scales showing the highest correlations to the Picq and Vayer test were Picture completion followed by Mazes (Table 4).
Sub-scales of the verbal scale WPPSI |
Sub-scales performance scale WPPSI |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Scales Picq and Vayer test |
Vocabulary |
Comprehension |
Sentences |
Information |
Similarities |
Arithmetic |
Picture completion |
Mazes |
Block design |
Copying geometric design |
Animal house |
Oculo-Manual Coordination (OMC) |
- |
- |
- |
- |
- |
- |
0.472 |
0.271 |
- |
- |
0.382 |
Dynamic Coordination (DC) |
- |
- |
0.265 |
0.266 |
- |
- |
0.294 |
0.287 |
- |
- |
- |
Postural Control And Balance (PCB) |
0.378 |
- |
0.328 |
0.283 |
- |
0.337 |
- |
0.389 |
- |
- |
- |
Body Control (BC) |
0.399 |
- |
0.363 |
0.362 |
- |
- |
0.367 |
0.347 |
0.296 |
- |
- |
Perceptual Organization (PO) |
- |
- |
- |
- |
- |
- |
0.27 |
- |
- |
- |
- |
Projective Drawing (PD) |
- |
- |
- |
- |
- |
- |
0.292 |
0.267 |
- |
0.465 |
0.293 |
Language (Le) |
0.493 |
0.479 |
0.451 |
0.384 |
0.32 |
- |
0.332 |
- |
- |
- |
- |
Speed (SP) |
- |
- |
- |
- |
- |
- |
- |
0.33 |
- |
- |
- |
Body Schema (BS) |
0.334 |
- |
- |
- |
- |
0.274 |
0.479 |
- |
0.398 |
0.335 |
- |
Table 4 Correlation coefficients between the scales of the Picq and Vayer test and the sub-scales of the WPPSI*
*Only statistically significant correlation coefficients are shown (p<0.05).
HC is the major preventable cause of learning disorders during childhood. Even though hormone replacement therapy reduces the risk of mental retardation, follow-up studies have reported an association between the CH initial conditions and delays or subtle manifestations of psycho-motor function disorders that, in the most severe cases, prevail until School-age and are associated to low academic performance. This can be explained by a prolonged exposure to low concentrations of thyroid hormone during the fetal period and its impact on the development of motor function and complex cognitive disorders, mainly in balance, visuospatial deficits, memory deficits, in fine motricity, and attention problems. In this study, athyrosic children showed, on average, lower scores for all the assessed sub-scales; these results are in agreement with those from other studies.1,20,21
From our results, the psychomotricity basic ability that was best related to GIQ and PIQ was Body schema, i.e., the organization of all sensations on the body (mainly tactile, visual and proprioceptive) regarding to data from the outside world4 It has been reported that after 2 years of age, the child organizes, structures, and integrates the elements and factors resulting from internal and external perceptions until they develop the perception of the whole body, thus achieving the construction of a true body schema at the age of 5 years old. Garaigordobil obtained similar results in children between 7.5 and 8 years old; these children, who showed a high level of body schema recognition, also showed adequate intelligence levels, both in VIQ and PIQ. A child that does not recognize their own body parts, will show difficulties to attain adequate coordination between what they observe and express, e.g., through writing or expressive language; hence the importance of promoting the development of body consciousness.16
On the other hand, a significant relation between projective drawing and the GIQ and PIQ was found. Graphic activity during preschool could be deemed to be a complex behavior, from which people expresses and communicates by means of different types of written languages or a number of visual forms33.The child first draws their body statically and then, according to their kinetic experiences, dynamically -showing some movement- within their experiential context; thus evidencing their nervous system maturation, their movements and expressions inventory, their personal identity, and will progressively interpret their social reality. Contained in this response resides the level of structuration of their body schema, as for their levels of attention and observation; their attitude, coordination, and affections; the use of their graphic arm; and the space and time where experiences occurring inside a three-dimensions space are to be limited to a two-dimensions space.34,35
Other elements of psychomotricity related to the IQ were postural control and balance. It has been established that the relations between attention and body consciousness are affected when the child have difficulties in the cognitive areas, expressed through omissions regarding to the orientation of body segments and difficulties to copy patterns that are presented to them. It has also been reported that coordination and balance might have an impact on the results from cognitive tests. When balance is defective, it takes more energy than necessary, and the child shows fatigue, attention disorders, motor skills disorder (clumsiness), muscle spasms, imprecisions, and synkinesias. Balance depends on the postural-tonic control, and it has also an impact on the coordination and organization of visual functions, thus losing harmony, precision and efficacy.4,36 However, it should be noted that, even though the highest correlation was observed with the Mazes sub-scale, all of the remaining sub-scales (4) showing a correlation belong to the Verbal scale. We have no further explanation than these processes being affected parallelly without the necessary existence of a subordination or dependency of any kind.
In a follow-up study carried out with 133 children, Kooistra et al.37 observed that athyrosic children at the age of 9 1/2 years old showed borderline intelligence and motor problems, mainly in balance. The authors attribute this finding to the effects that low thyroid hormone concentrations have on the ontogeny of the cerebellum.37 On the other hand, a recent study found that CH severity did not affect the motor or the intellectual development in children of 9.6+3.9 years old with CH. However, the time required to bring the levels of free T4 to a normal range was indeed related to poor balance.38
During the age of preschool, verbalization and internalization of language will be the instrument allowing the child to integrate all of the factors constituting their body schema and to control the thoughts directing their motor behavior, resulting in reflexive thinking and the ability to anticipate movement4. Our results showed a strong association between LE and the VIQ and GIQ. One study reported that prolonged exposure to low concentrations of thyroid hormones is a major risk factor for the development of language disorders.39 Álvarez-González et al.1 carried out an study in Cuba with 100 children diagnosed with congenital hypothyroidism, whose cognitive performance was assessed periodically. They found that the duration of fetal hypothyroidism was related to postural control, and the initial biochemical severity to the development of language.1 Gejão and Lamônica40 found that children with CH were at a higher risk of developing expressive language developmental disorders, although they observed a trend towards an adequate performance in children diagnosed and treated promptly, who were administered with high doses of thyroxine at treatment initiation.40
Oculo-Manual coordination plays an important role, either as a participative element or as a predictor for the later cognitive functions. Data indicate that this factor is acquired during early childhood and it is mastered at the age of preschool, and it, along with development, allows movements and perception to achieve complex levels of functioning and help the child to accomplish school requirements. Some studies have found that this function is significantly and inevitably reduced in children with CH, i.e., this deficit is already preset and out of the direct influence of the severity of the disease and of the treatment scope.1,41 However, our results show that children in the Athyrosic group under perform in the Oculo-Manual item versus children in the Ectopia group.
Results from other studies show that the psychomotor development in preschoolers is the major precedent of the performance functions of children. Moreover, to know this sort of associations enable professionals to specify handling and intervention strategies during this stage in order to prevent or lower the risks for children to develop learning problems. On the other hand, they allow us to recognize the necessity that during early childhood, educators consider planning an environment for learning.
None.
Authors declare that there is no conflict of interest

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.