International Journal of
eISSN: 2574-9889


Case Report Volume 5 Issue 5
Department of Obstetrics and Gynaecology, Mater Dei Hospital, Malta
Correspondence: Silvaine M Dalli, Department of Obstetrics and Gynaecology, Mater Dei Hospital, Malta
Received: July 11, 2019 | Published: September 10, 2019
Citation: Dalli SM, Fenech A, Abela E, et al. Management of hodgkin lymphoma diagnosed in the first trimester. Int J Pregn & Chi Birth. 2019;5(5):167-171. DOI: 10.15406/ipcb.2019.05.00169
Hodgkin lymphoma is a tumour of the lymphatic system which decreases the body’s abilities to fight an infection. In this case report we present a 23 year old lady who was diagnosed with Hodgkin lymphoma during early pregnancy. She was started on ABVD (adriamycin, bleomycin, vinblastine and dacarbazine) during the second trimester to decrease the risk of foetal abnormalities and was followed up with regular growth scans. Delivery at 32 weeks gestation by C-section was successful with the birth of a healthy baby. Imaging after treatment showed complete regression of the disease.
Keywords: lymphoma, preterm birth, pregnancy, organogenesis
MRI, magnetic resonance imaging; CT, computed tomography.
Hodgkin lymphoma is a tumour of the lymphatic system which impairs the body’s ability to fight infections. It usually presents with lethargy, night sweats, unexplained weight loss, painless enlarged lymph nodes and loss of appetite. It can be subdivided into two: the classical type which is characterised by Reed-Sternberg cells and the lymphocyte predominant form which is characterised by large popcorn cells. It is commonest between the ages of 15 and 30 and is associated with an infection by Epstein Barr virus and a compromised immune system.1
Cancer is diagnosed in around 8 pregnancies per 100,000 births,2 with Hodgkin lymphoma being the fourth commonest cancer to be diagnosed. It has an estimated incidence of 1 in 1000 to 1 in 3000.3 Treatment is usually successful with a 90-95% 5 year survival rate in stage I or II disease. A large study showed that women suffering from Hodgkin lymphoma during pregnancy are at increased risk of developing venous thromboembolism, needing a blood transfusion post-partum and of having a preterm birth.2 In this case the diagnosis was made early in pregnancy, but due to the legal status in the country a termination was not an option, and the mother decided to the keep the baby irrespective of the legal status. The decision to postpone treatment until the second trimester was taken to limit the effect that the drugs could have on organogenesis.
A 23 year old lady with no past medical history of note presented to the ear, nose and throat department with a history of masses in her neck, night sweats and weight loss. She was noted to have multiple enlarged cervical lymph nodes on the left side of her neck which were biopsied at 7 weeks gestation. The biopsy showed aggregates of large neoplastic cells with large lobulated nuclei, prominent inclusion-like eosinophilic nucleoli and abundant amphophillic cytoplasm. Reed-Sternberg cells were also noted and were accompanied by a mixed inflammatory cell infiltrate.Immunohistochemistry as performed and neoplastic cells were found to be CD15 and CD30 positive. This was conclusive of a diagnosis of classical Hodgkin lymphoma – nodular sclerosis type.4 Imaging was also performed at the time and it showed multiple enlarged lymph nodes in the left cervical, bilateral supraclavicular, axillary and supra-mediastinal lymph nodes as seen in Figures 1&2.
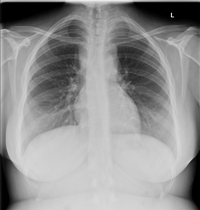
Figure 1 Chest X-ray at diagnosis showing loss of the aorto-pulmonary window and widening of the right paratracheal stripe.
The case was managed with the haematological team, who carried out a trephine bone marrow biopsy which showed that there was no infiltration to bone marrow. A staging MR scan was carried out at this stage, which excluded the disease from the liver and spleen. She was also being followed up from the obstetric aspect at the same time. She had a booking visit at 8 weeks gestation at which point an ultrasound confirmed the presence of a foetus with foetal heart pulsations and a crown-rump length of 1.65cm. Virology, including HIV which was negative and, renal function a complete blood count liver function tests, lactate dehydrogenase, uric acid CRP were all done and were within normal limits. The case was discussed in a multidisciplinary team and it was decided to postpone chemotherapy until the second trimester. In fact, she was started on ABVD at 14 weeks gestation. In view of the haematological disease it was decided to start her on low molecular weight heparin to decrease the risk of venous thromboembolic events. She responded well to treatment as was evidenced by the MR scan done after treatment which showed a significant reduction in the lymphadenopathy, as seen in Figure 3. Throughout treatment she had regular growth scans as well as a 24 week anomaly scan which was normal.
After discussion within the multidisciplinary team, it was decided to plan for delivery by elective C-section between 34 and 36 weeks gestation. However, she was admitted at 32 weeks for observation after it was noted that foetal growth was suboptimal. Ultrasound showed that there was an element of intrauterine growth retardation with an umbilical artery doppler above the 90th centile and oligohydramnios. Thus, delivery was expedited and a C-section was done at 32+4 weeks gestation. The baby had a birth weight of 1.23Kg and needed to be admitted at the neonatal intensive care unit due to the growth restriction. ABVD was stopped for 2 weeks post-partum after which it was resumed.Follow up MR imaging after the second and fourth cycles revealed response to therapy in the former and resolution of the disease in the latter. A PET-CT carried out one year after diagnosis confirmed complete regression of the disease. Neonatal outcome was good and the baby has had normal developmental milestones and no complications as a result of maternal treatment during pregnancy.
Management
With regards to the oncological treatment during pregnancy, the patient received ABVD and she was closely followed up for signs and symptoms of toxicity as seen in more detail in Table 1. The ECOG performance status was used to assess the patient’s condition after treatment. This is a measure of assessing the patient’s fitness after treatment and ranges from a scale of 0 to 5, with 0 meaning an active patient who can carry out all pre-disease activities without restriction and 5 being dead.
Gestation |
Cycle |
Toxicities following cycle |
|
13+5 |
1A |
|
Normal spirometry (baseline) |
16+4 |
1B |
ECOG 0; nausea – no other toxicities |
|
19+1 |
2A |
ECOG 0; no toxicities |
Normal spirometry |
20+5 |
2B |
ECOG 0; no toxicities |
|
23+5 |
3A |
ECOG 0; mild phlebitis following dacarbazine |
Normal spirometry |
26 |
3B |
ECOG 0; no toxicities |
|
|
4A |
ECOG 0; no toxicities |
Normal spirometry |
30 |
4B |
ECOG 0; no toxicities |
|
Table 1 Follow up of the patient for toxicity
An initial MRI showed multiple enlarged lymph nodes as seen in image 2 and given these findings a decision to start treatment in the second trimester was taken. She was also followed up by further imaging to assess her response to treatment. An MRI which was done after 2 cycles of chemotherapy showed partial response, whilst a PET scan done after 4 cycles of treatment showed complete response, as can be seen in image 3. She also continued treatment post-partum and received two further cycles of chemotherapy. Resumption of chemotherapy was postponed by two weeks to allow for recovery after the C-section.
A prophylactic dose of 40mg of low molecular weight heparin was given throughout pregnancy. She was also receiving support medication in tandem with the chemotherapy, and these included allopurinol 300mg daily for 2 weeks, co-trimoxazole 960mg three times a week, ondansetron 8mg as needed up to a maximum of three times a day and dexamethasone. Aciclovir at a dose of 200mg three times a day and co-trimoxazole at a dose of 480 mg daily three times a week were used as prophylaxis whilst receiving chemotherapy. As per the SPC for aciclovir, it has been shown to be safe for use during pregnancy. Both trimethoprim and sulfamethoxazole cross the placenta and the safe use of these drugs in pregnancy has not been concluded yet. Co-trimoxazole was used in our patient because of the risk of Pneumocystis jiroveci pneumonia infection in patients with Hodgkin’s lymphoma.
The occurrence of cancer during pregnancy is a rare event. However, the incidence has increased because of an increase in maternal age at the time of the first pregnancy and currently stands at 1:1000 births.5 About 3.2% of patients suffering from Hodgkin lymphoma are pregnant,2 and being pregnant can complicate treatment.
There are no prospective trials for the management of Hodgkin’s lymphoma in pregnancy, however, there are several case reports, case series and case cohort studies showing excellent maternal and foetal outcomes following Hodgkin lymphoma treatment in pregnancy. In fact, a case-controlled study of 48 patients showed that they had long term survival comparable to those who were not pregnant at diagnosis, and similarly pregnancy outcome was not adversely affected with no metastases to the foetus or placenta.6
It is the fourth most commonly diagnosed malignancy in pregnancy and fortunately is one of the most curable tumours, especially if like in this case it is diagnosed early.7 However, pregnancy complicates treatment since chemotherapeutic agents must be avoided during the first trimester, during which organogenesis occurs, and radiotherapy should be avoided late in pregnancy due to the uterus’ proximity to the groin nodes.
Diagnosis should ideally be made by lymph node biopsy under local anaesthesia, and if no superficial lymph nodes are available, by biopsy under general anaesthesia.8 When general anaesthesia is given during pregnancy for non-obstetric surgery the rate of miscarriage is about 5.8% and prematurity affects 8.2% of pregnancies. Undergoing surgery during the first trimester is associated with a low rate of major birth defects.9
Once a histological diagnosis is obtained, there also needs to be staging which is done by Computed Tomography (CT) scanning. This was found to be safe in pregnancy as the dose that is administered is lower than that that causes severe congenital abnormalities.10 In our case a CT scan was used in the initial phase of staging, but thereafter magnetic resonance imaging (MRI) scanning was used to follow up the response to treatment. When doing MRI, research shows that ideally this is done gadolinium-free, since this can cross the placenta and increase the risk of the foetus developing rheumatologic or inflammatory skin conditions.11
Chemotherapeutic agents in pregnancy
Pregnancy affects the pharmacokinetics and pharmacodynamics of drugs which are administered since there are various changes in the body during pregnancy.12 Furthermore, drugs can also influence the growing foetus, especially cytotoxic drugs which can cross the placenta due to their low molecular weight.13
In this case, cytotoxic drugs could not be avoided as the diagnosis was made early in pregnancy and delaying treatment would not have been beneficial for either the mother or the foetus. However, treatment was started in the second trimester when the risk of spontaneous miscarriage and the development of congenital abnormalities due to chemotherapy would be reduced as the organogenesis period would have been over.14,15 When given at a later stage in pregnancy, the risk of stillbirth, preterm labour and intrauterine growth retardation with a low birth weight increases, so the patient was monitored closely with regular growth scans.16
ABVD
The British Society for Haematology (BSH) have included the management of Hodgkin lymphoma in pregnancy within the guidelines for classical Hodgkin lymphoma. The key BSH recommendations include ABVD as the protocol of choice in pregnancy and the postponement of radiotherapy until after delivery wherever possible.17 Bachanova & Connors18 have reviewed the published cases and shown that ABVD is the regimen of choice when multi-drug chemotherapy is planned. This is based upon the experience described in the comprehensive literature review, since evidence to support a chemotherapy recommendation in pregnant patients with Hodgkin lymphoma in very limited. Nine articles out of those identified were original publications describing chemotherapy used solely in pregnant patients with Hodgkin lymphoma.
Additionally, a review done in 2007 advises deferring treatment until the second trimester with close observation of the disease unless it is advanced or aggressive at diagnosis. In that case ,the advice is to start chemotherapy and advise termination.19 Two case series reported no adverse foetal outcomes in 13 patients treated with standard ABVD in the first, second or third trimesters.20,21 Another case series reported 15 pregnant patients who received ABVD, MOPP or COPP in any trimester and delivered healthy infants.21 The survival of 21 pregnant patients with Hodgkin lymphoma treated with MOPP chemotherapy, radiotherapy or a combination of both was similar to that of matched non-pregnant controls.22 The case reports cited the used of single agent vinblastine during the first trimester with no adverse effects of the pregnancy nor foetal development.
Foetal implications
Data regarding the outcome of children having received in utero chemotherapy is reassuring, even though the studies and number of patients assessed are limited.23,24 It is only the administration of chemotherapy in the embryonal stage that can lead to miscarriage.5 The association between chemotherapy and embryo-foetal toxicity explains the recommendation to delay the start of chemotherapy until after the end of the first trimester.14
Doxorubicin is the anthracycline in the ABVD regimen, which is safe to use in the second and third trimesters, although it is the least studied in this group. A case series of nine patients showed that when given after 14 weeks gestation no major malformations occurred, except for a single case of syndactyly which was treated surgically.21 Another case series with ten cases had similar findings, except for a case of intrauterine growth retardation.25 Aviles et al.,26 have assessed 81 children whose mothers were exposed to anthracycline-containing chemotherapy regimens during pregnancy. This is the first paper reporting on cardiac follow up for up to 20 years post exposure to anthracyclines in utero. There was no clinical or echocardiogram evidence of late cardiac toxicity. The children were assessed every 5 years after birth until 29 years of age. The mean dose of doxorubicin used was 355mg/m2. Our patient received doxorubicin at a dose of 25mg/m2 every 2 weeks with a total dose of 200mg/m2 administered during pregnancy. Amant et al.,27 also found no structural abnormalities in children who had been exposed to anthracyclines in utero. A retrospective analysis carried out by Germann et al.,28 regards anthracyclines during pregnancy have reported 2 cases of cardiac toxicity with the use of anthracyclines.
The low transplacental transfer of doxorubicin and epirubicin is reassuring regarding fetal toxicity and long-term effects. However, the unknown susceptibility of the fetal tissues to even low cytotoxic drug concentrations needs to be further explored.29 The fetal heart may be drug-sensitive since anthracyclines induce a dose-related cardiotoxicity.30 To date, case series do not show impaired heart function or morphology in children after prenatal exposure to anthracycline-based chemotherapy.26,31 The low levels of anthracyclines in the foetal heart may contribute to a favourable cardiac outcome in the offspring.
Adriamycin and bleomycin are both anthracycline antibiotics which act by interfering with DNA synthesis. It is thought that they can contribute to cardiac abnormalities as it can lead to dose-related toxicity with myocardial damage secondary to free radical damage.32 Anthracyclines have been demonstrated to cross the placenta.33 Ryu et al have assessed the pharmacokinetics of doxorubicin in mid-late pregnant subjects34 and their results showed a significantly reduced creatinine clearance of doxorubicin at the 48 and 72 hour blood and urine sampling, however, further studies are required to determine whether different dosage strategies are required in pregnant patients. Germann et al.,28 performed a literature review regarding the embryo-foetal outcome following anthracycline treatment during pregnancy. Anthracyclines interact with the DNA-topoisomerase II complex leading to its disruption. Only low concentrations of anthracyclines have been found in foetal tissues, however, topoisomerase IIα is overexpressed in rapidly developing tissues. The low transfer value of doxorubicin is explained by the high molecular weight of the drug and the hydrophilic nature of doxorubicin which leads to a slow placental transfer. They recommend administration of anthracycline from the second trimester to prevent foetal malformations. The risk of embryo-foetal toxicity is however low, especially with doses of less than 70mg/m2.
Data regarding the embryo-foetal toxicity of bleomycin is very limited, with reports available in the literature documenting the use of much lower doses of bleomycin than those administered with the ABVD regimen. This was not associated with adverse outcomes.35 Cardonick et al have shown no significant differences in cognitive skills, academic achievement or behavioural difference between children exposed to chemotherapy in utero and the unexposed.36 Vinblastine, a plant alkaloid which is less teratogenic than others since it is bound to protein. It has been found to increase the risk of intrauterine growth restriction, preterm delivery and pre-eclampsia when used during the second and third trimesters.37 Dacarbazine is the least studied alkylatin in pregnancy and its use has been reported as part of a multi-drug regimen therefore the foetal adverse outcome cannot be attributed to dacarbazine alone. Congenital abnormalities, foetal death and foetal prematurity have all been reported with regimens including dacarbazine.38
Maternal complications
Being pregnant increases the risk of thromboembolism and it is a leading cause for death during pregnancy, but having a haematologic or gynaecologic malignancy further increases this risk.39–41 Thus, the patient was put on low molecular weight heparin to decrease the risk of thromboembolic events which could further complicate the pregnancy.
The chemotherapeutic agents used can also pose a risk to the mother. Bleomycin can lead to the development of pneumonitis during treatment and rarely can also lead to development of pulmonary fibrosis.42 Anthracyclines can also cause a cardiomyopathy, especially left ventricular dysfunction, which is dose dependent and if severe enough can precipitate heart failure later in pregnancy when the cardiovascular changes that occur during the pregnancy stress the heart more; during the third stage of labour there is an influx of blood in the system with an associated increase in cardiac output.43,44 Having survived Hodgkin lymphoma, the patient is also at risk of developing secondary malignancies with solid tumours like breast, lung and gastrointestinal tumours making up to 70-80% of secondary malignancies in such patients.45 Thus, she should be thoroughly assessed for the presence of such secondary tumours prior to getting pregnant again.
Although a rare disease in pregnancy, it is important that management for such a disease is holistic and multidisciplinary to ensure a positive outcome for both the mother and the fetus. Understandably, such a diagnosis would be distressing to the patient and it is essential that she is supported throughout the whole process.
None.
The author declares there are no conflicts of interest.
None.

©2019 Dalli, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.